Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 , Br
, Br
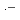 , and Br
, and Br
 in aqueous solutions
in aqueous solutionsLin, M.; Archirel, P.*; Van-Oanh, N. T.*; Muroya, Yusa*; Fu, H.*; Yan, Y.*; Nagaishi, Ryuji; Kumagai, Yuta; Katsumura, Yosuke*; Mostafavi, M.*
Journal of Physical Chemistry A, 115(17), p.4241 - 4247, 2011/04
Times Cited Count:14 Percentile:44.6(Chemistry, Physical)The absorption spectra of Br
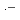 and Br
and Br
 in aqueous solutions are investigated by pulse radiolysis techniques from room temperature to 380 and 350
in aqueous solutions are investigated by pulse radiolysis techniques from room temperature to 380 and 350 C, respectively. The weak temperature effect on the absorption spectra of Br
C, respectively. The weak temperature effect on the absorption spectra of Br
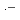 and Br
and Br
 is because in these two systems, the transition occurs between two valence states. We performed classical dynamics of hydrated Br
is because in these two systems, the transition occurs between two valence states. We performed classical dynamics of hydrated Br system at 20 and 300
system at 20 and 300 C under pressure of 25 MPa. This shows that the first water shell is strongly bound to the anion whatever the temperature. The first two water shells form a cavity of a roughly spherical shape around the anion. By TDDFT method, we calculated the absorption spectra of hydrated Br
C under pressure of 25 MPa. This shows that the first water shell is strongly bound to the anion whatever the temperature. The first two water shells form a cavity of a roughly spherical shape around the anion. By TDDFT method, we calculated the absorption spectra of hydrated Br at two temperatures and we compared the results with the experimental data.
at two temperatures and we compared the results with the experimental data.
 , Br
, Br
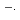 and Br
and Br
 in aqueous solutions
in aqueous solutionsLin, M.; Muroya, Yusa*; Katsumura, Yosuke*; Archirel, P.*; Van-Oanh, N. T.*; Fu, H.*; Yan, Y.*; Nagaishi, Ryuji; Kumagai, Yuta; Mostafavi, M.*
no journal, ,
The absorption spectra of Br
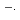 and Br
and Br
 in aqueous solutions are investigated by pulse radiolysis techniques from room temperature to 380 and 350
in aqueous solutions are investigated by pulse radiolysis techniques from room temperature to 380 and 350 C, respectively. The weak temperature effect on the absorption spectra of Br
C, respectively. The weak temperature effect on the absorption spectra of Br
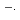 and Br
and Br
 is because in these two systems, the transition occurs between two valence states. We performed classical dynamics of hydrated Br
is because in these two systems, the transition occurs between two valence states. We performed classical dynamics of hydrated Br system at 20 and 300
system at 20 and 300 C under pressure of 25 MPa. This shows that the first water shell is strongly bound to the anion whatever the temperature. The first two water shells form a cavity of a roughly spherical shape around the anion. By TDDFT method, we calculated the absorption spectra of hydrated Br
C under pressure of 25 MPa. This shows that the first water shell is strongly bound to the anion whatever the temperature. The first two water shells form a cavity of a roughly spherical shape around the anion. By TDDFT method, we calculated the absorption spectra of hydrated Br at two temperatures and we compared the results with the experimental data.
at two temperatures and we compared the results with the experimental data.
 , Br
, Br
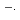 , Br
, Br
 in aqueous solutions at elevated temperatures
in aqueous solutions at elevated temperaturesLin, M.; Muroya, Yusa*; Katsumura, Yosuke*; Archirel, P.*; Van-Oanh, N. T.*; Nagaishi, Ryuji; Kumagai, Yuta; Mostafavi, M.*
no journal, ,
The temperature dependent absorption spectra of Br , Br
, Br
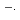 and Br
and Br
 have been investigated by pulse radiolysis or steady-state irradiation. The spectra of Br
have been investigated by pulse radiolysis or steady-state irradiation. The spectra of Br
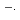 and Br
and Br
 exhibit almost no shift at the absorption maximum, while that of Br
exhibit almost no shift at the absorption maximum, while that of Br shows a red-shift with increasing temperature. Quantum chemical calculations have been also performed to unveil the nature of the spectral shift of Br
shows a red-shift with increasing temperature. Quantum chemical calculations have been also performed to unveil the nature of the spectral shift of Br .
.