Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Emori, Tatsuya; Kitatsuji, Yoshihiro; Ban, Yasutoshi
JAEA-Technology 2024-025, 20 Pages, 2025/03
Radioisotope Thermoelectric Generators (RTGs) using the decay heat of Pu-238 has been applied for outer planet missions far from Jupiter, where solar power is limited. However, no facilities are available to produce Pu-238 for space probes in Japan. Moreover, the use of nuclear materials for the space exploration is difficult in term of the regulation. Thus, we focused on Am-241 whose half-life is around 432 years as an alternative heat source for RTGs. This report describes the procedure of separating Am-241 decayed from Pu-241 in aged plutonium oxide. Two experiments were performed: one using solid-liquid extraction and the other combining liquid-liquid extraction and solid-liquid extraction. Packed columns were used in the experiments, with their number reduced by less than one-fifth in the latter experiment compared to the former. Furthermore, the time required for separation in the latter experiment was less than half that of the former. We performed the separation experiments six times, collecting a total of approximately 0.43 g of Am-241 as an oxalate salt.
Suzuki, Hideya*; Ban, Yasutoshi
Journal of Nuclear Science and Technology, 62(2), p.157 - 166, 2025/02
Times Cited Count:0 Percentile:0.00(Nuclear Science & Technology)Nakamura, Satoshi; Ishii, Sho*; Kato, Hitoshi*; Ban, Yasutoshi; Hiruta, Kenta; Yoshida, Takuya; Uehara, Hiroyuki; Obata, Hiroki; Kimura, Yasuhiko; Takano, Masahide
Journal of Nuclear Science and Technology, 62(1), p.56 - 64, 2025/01
Times Cited Count:1 Percentile:51.66(Nuclear Science & Technology)A dissolution method for analyzing the elemental composition of fuel debris using the sodium peroxide (Na O
O ) fusion technique has been developed. Herein, two different types of simulated debris materials (such as solid solution of (Zr,RE)O
) fusion technique has been developed. Herein, two different types of simulated debris materials (such as solid solution of (Zr,RE)O and molten core-concrete interaction products (MCCI)) were taken. At various temperatures, these debris materials were subsequently fused with Na
and molten core-concrete interaction products (MCCI)) were taken. At various temperatures, these debris materials were subsequently fused with Na O
O in crucibles, which are made of different materials, such as Ni, Al
in crucibles, which are made of different materials, such as Ni, Al O
O , Fe, and Zr. Then, the fused samples are dissolved in nitric acid. Furthermore, the effects of the experimental conditions on the elemental composition analysis were evaluated using inductively coupled plasma-atomic emission spectroscopy (ICP-AES), which suggested the use of a Ni crucible at 923 K as an optimum testing condition. The optimum testing condition was then applied to the demonstration tests with Three Mile Island unit-2 (TMI-2) debris in a shielded concrete cell, thereby achieving complete dissolution of the debris. The elemental composition of TMI-2 debris revealed by the proposed dissolution method has good reproducibility and has an insignificant contradiction in the mass balance of the sample. Therefore, this newly developed reproducible dissolution method can be effectively utilized in practical applications by dissolving fuel debris and estimating its elemental composition.
, Fe, and Zr. Then, the fused samples are dissolved in nitric acid. Furthermore, the effects of the experimental conditions on the elemental composition analysis were evaluated using inductively coupled plasma-atomic emission spectroscopy (ICP-AES), which suggested the use of a Ni crucible at 923 K as an optimum testing condition. The optimum testing condition was then applied to the demonstration tests with Three Mile Island unit-2 (TMI-2) debris in a shielded concrete cell, thereby achieving complete dissolution of the debris. The elemental composition of TMI-2 debris revealed by the proposed dissolution method has good reproducibility and has an insignificant contradiction in the mass balance of the sample. Therefore, this newly developed reproducible dissolution method can be effectively utilized in practical applications by dissolving fuel debris and estimating its elemental composition.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Suzuki, Tatsuya*
Solvent Extraction Research and Development, Japan, 32(1), p.21 - 29, 2025/00
The magnitude of the masking effect of carboxylic, amic-acidic, and amidic compounds through Ln and Am extractions by tetraoctyl diglycolamide (TODGA) is compared. The compounds used are diglycol, ethylenediamine, diethylenetriamine-type, and two other amides (dioxaoctane diamide and nitrylotriacetamide). The results show that below pH 1.2, where carboxylic acids are less dissociated, amide O atoms have higher reactivity with lanthanides than O atoms in carboxyl groups. From observing the Ln patterns (D(Ln) vs. their atomic number), the compounds primarily show high reactivity, with middle and heavy Ln having a higher charge density than light Ln. Four amide compounds are employed in this work. Those with tertiary amine N atoms have pH dependence on D(Ln) due to protonation and dissociation from amine N atoms.
Minowa, Kazuki*; Watanabe, So; Nakase, Masahiko*; Takahatake, Yoko; Miyazaki, Yasunori; Ban, Yasutoshi; Matsuura, Haruaki*
Nuclear Instruments and Methods in Physics Research B, 556, p.165496_1 - 165496_6, 2024/11
Times Cited Count:0 Percentile:0.00(Instruments & Instrumentation)In this study, X-ray absorption near edge structure (XANES) spectral analysis and column experiments were used to verify the selectivity of rare earth (RE) ions by alkyl diamide amine (ADAAM) adsorbent. In addition, the interactions between the N atoms of ADAAM and RE ions were evaluated to determine whether any of the RE ions are a valid simulant for developing a mutual separation process for minor actinides (MAs) in highly radioactive liquid waste. It was confirmed that La and Ce interacted with the amine N atom of ADAAM and they showed a peak shift of the N-K edge XANES spectrum; this finding suggested that a soft interaction is an essential factor influencing ion selectivity. Therefore, the selection factor of RE ions by ADAAM adsorbent was similar to that of MAs. It was concluded that RE ions are reasonable species to simulate MAs.
Ban, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsubata, Yasuhiro
Proceedings of International Conference on Nuclear Fuel Cycle (GLOBAL2024) (Internet), 4 Pages, 2024/10
A continuous counter-current extraction experiment was performed by mixer-settler extractors to recover minor actinides (MA; Am and Cm) from high-level liquid waste. Using hexaoctyl nitrilotriacetamide (HONTA) as an extractant, 0.17 g of MA was recovered in a MA fraction.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Suzuki, Hideya*
Journal of Nuclear Science and Technology, 61(7), p.883 - 893, 2024/07
Times Cited Count:4 Percentile:59.85(Nuclear Science & Technology)The mutual separation of Am and Cm is conducted using an alkyl-diamide amine (ADAAM) extractant. ADAAM exhibits extremely high separation factor with respect to Am and Cm separation (5.9) in a nitric acid- -dodecane system. The batch-wise multistage extractions are performed using a system containing 0.2 M ADAAM and 1.5 M nitric acid. In this multistage extraction, an organic solvent give 96.5% and 1.06% yields of Am and Cm. After the mutual separation of Am and Cm, an additional extraction step is included to reduce the volumes of these aqueous and organic phases. Taking these steps, Am and Cm can be recovered in just two or three stages in the aqueous phases.
-dodecane system. The batch-wise multistage extractions are performed using a system containing 0.2 M ADAAM and 1.5 M nitric acid. In this multistage extraction, an organic solvent give 96.5% and 1.06% yields of Am and Cm. After the mutual separation of Am and Cm, an additional extraction step is included to reduce the volumes of these aqueous and organic phases. Taking these steps, Am and Cm can be recovered in just two or three stages in the aqueous phases.
 -hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cell
-hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsubata, Yasuhiro
Solvent Extraction Research and Development, Japan, 31(1), p.1 - 11, 2024/00
A demonstration test was performed to separate minor actinides (MA; Am and Cm) by  -hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm
-hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm in
in  -dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were
-dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were  99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Kinoshita, Ryoma; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
Analytical Sciences, 39(9), p.1575 - 1583, 2023/09
Times Cited Count:3 Percentile:35.90(Chemistry, Analytical)Extraction of Rh from HCl can be performed by NTAamide(C6) (hexahexyl-nitrilotriacetamide) and other related compounds into n-dodecane. We use ion-pair extraction of anionic species of Rh-chloride and protonated extractant. Rh behave as anion in hydrochloric acid and the tertiary nitrogen atom in extractant may be protonated to produce the quaternary amine in acidic condition. From the present work, the maximum distribution ratio of Rh(III) is 16. The D(Rh) values are changeable during preparation of the aqueous solutions because different Rh-Cl-H O complexes are formed in HCl media and show the slow exchange rate between Cl and H
O complexes are formed in HCl media and show the slow exchange rate between Cl and H O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl
O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl (H
(H O)
O) and RhCl
and RhCl (H
(H O)
O)
 exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
Suzuki, Hideya*; Ban, Yasutoshi
Analytical Sciences, 39(8), p.1341 - 1348, 2023/08
Times Cited Count:4 Percentile:46.80(Chemistry, Analytical)The Japan Atomic Energy Agency (JAEA) has proposed the Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation (SELECT) process by solvent extraction as a new separation technology to recover minor actinides (MA) from high-level liquid waste (HLLW) produced by spent fuel reprocessing. The MA separation in the SELECT process comprises the batch recovery of MA and rare earths (RE) from HLLW, MA/RE separation, and Am/Cm separation. Three highly practical extractants are used in the MA separation. Furthermore, this flow configuration facilitates the preparation of nitric acid concentrations in the aqueous phase. However, the separation factor between Cm and Nd in the MA/RE separation is small (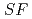
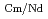 = 2.5), requiring many extraction stages for continuous extraction in a mixer-settler. Therefore, this study investigated the separation of only Am from an aqueous nitric acid solution containing MA (Am and Cm) and RE using an organic phase mixed with two extractants alkyl diamideamine with 2-ethylhexyl alkyl chains (ADAAM(EH)) and hexa-n-octylnitrilotriacetamide (HONTA) used in the SELECT process. Under high-concentration nitric acid conditions, Am and La, Ce, Pr, Nd (light lanthanides) were extracted in the ADAAM(EH) + HONTA mixed solvent, whereas Cm, medium, and heavy lanthanides, and Y were partitioned in the aqueous phase. Subsequently, only light lanthanides could be back extracted from the ADAAM(EH) + HONTA mixture solvent containing Am and light lanthanides in low nitric acid concentrations. Furthermore, Am could be easily stripped with 0.2 M or 5 M nitric acid. This method does not require the mutual separation of Cm and Nd, which have low separation factors. Am can be efficiently separated by one extraction and two back-extractions, reducing the number of steps in the SELECT process.
= 2.5), requiring many extraction stages for continuous extraction in a mixer-settler. Therefore, this study investigated the separation of only Am from an aqueous nitric acid solution containing MA (Am and Cm) and RE using an organic phase mixed with two extractants alkyl diamideamine with 2-ethylhexyl alkyl chains (ADAAM(EH)) and hexa-n-octylnitrilotriacetamide (HONTA) used in the SELECT process. Under high-concentration nitric acid conditions, Am and La, Ce, Pr, Nd (light lanthanides) were extracted in the ADAAM(EH) + HONTA mixed solvent, whereas Cm, medium, and heavy lanthanides, and Y were partitioned in the aqueous phase. Subsequently, only light lanthanides could be back extracted from the ADAAM(EH) + HONTA mixture solvent containing Am and light lanthanides in low nitric acid concentrations. Furthermore, Am could be easily stripped with 0.2 M or 5 M nitric acid. This method does not require the mutual separation of Cm and Nd, which have low separation factors. Am can be efficiently separated by one extraction and two back-extractions, reducing the number of steps in the SELECT process.
Sano, Yuichi; Sakamoto, Atsushi; Miyazaki, Yasunori; Watanabe, So; Morita, Keisuke; Emori, Tatsuya; Ban, Yasutoshi; Arai, Tsuyoshi*; Nakatani, Kiyoharu*; Matsuura, Haruaki*; et al.
Proceedings of International Conference on Nuclear Fuel Cycle; Sustainable Energy Beyond the Pandemic (GLOBAL 2022) (Internet), 4 Pages, 2022/07
We developed a hybrid MA(III) recovery process combining MA(III)+Ln(III) co-recovery flowsheet by solvent extraction with TBP and MA(III)/Ln(III) separation flowsheet by simulated moving bed chromatography using HONTA impregnated adsorbents with large particle size porous silica support.
Toigawa, Tomohiro; Kumagai, Yuta; Yamashita, Shinichi*; Ban, Yasutoshi; Matsumura, Tatsuro
UTNL-R-0502 (Internet), 2 Pages, 2022/04
This report summarizes the results obtained in FY2020 at the Electron Linac Facility of the University of Tokyo. The radiolysis process of  -hexaoctyl nitrilotriacetamide (HONTA), which is expected to be used as an extractant in a separation process for minor actinides, diluted in dodecane was investigated by pulse radiolysis experiments. The radical cation and the triplet-excited state of HONTA were observed in the nanosecond time region. The transition from the radical cation to the triplet excited state was slowed down by adding electron scavengers, and further, the reactivity of the triplet excited state was also suppressed.
-hexaoctyl nitrilotriacetamide (HONTA), which is expected to be used as an extractant in a separation process for minor actinides, diluted in dodecane was investigated by pulse radiolysis experiments. The radical cation and the triplet-excited state of HONTA were observed in the nanosecond time region. The transition from the radical cation to the triplet excited state was slowed down by adding electron scavengers, and further, the reactivity of the triplet excited state was also suppressed.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Separation Science and Technology, 57(16), p.2543 - 2553, 2022/00
Times Cited Count:5 Percentile:28.02(Chemistry, Multidisciplinary)The mutual separation of actinides (An) from lanthanides (Ln) using the masking agent of DTPA (diethylenetriamine-pentaacetic acid) or DTBA (diethylenetriamine-triacetic acid-bis(diethylacetamide)) in the aqueous phase through DGA extraction, referring TALSPEAK method, is focused. We investigate to obtain the same separation performance using commercially available DTPA on that using DTBA. In this work, we select lactic acid (LA) of pH buffer from 10 organic acids and ethylenediamine (ED) for the pH adjustment. Almost the same D and SF values are obtained among the conditions: TODGA-DTPA-LA-NaOH, TODGA-DTPA-LA-ED, and TODGA-DTBA-LA. The experimental results using batchwise multi-stage extractions show the average yields of Ln (La to Gd) and Am to be 3.73 and 98.1% in the aqueous phase using DGA-DTPA-LA-ED, to be 3.1 and 97.0% using DGA-DTPA-LA-NaOH, and to be 1.61 and 98.7% using DGA-DTBA-LA.
Sugawara, Takanori; Moriguchi, Daisuke*; Ban, Yasutoshi; Tsubata, Yasuhiro; Takano, Masahide; Nishihara, Kenji
JAEA-Research 2021-008, 63 Pages, 2021/10
This study aims to perform the neutronics calculations for accelerator-driven system (ADS) with a new fuel composition based on the SELECT process developed by Japan Atomic Energy Agency because the previous studies had used the ideal MA (minor actinide) fuel composition without uranium and rare earth elements. Through the neutronics calculations, it is shown that two calculation cases, with/without neptunium, satisfy the design criteria. Although the new fuel composition includes uranium and rare earth elements, the ADS core with the new fuel composition is feasible and consistent with the partitioning and transmutation (P&T) cycle. Based on the new fuel composition, the heat removal during fuel powder storage and fuel assembly assembling is evaluated. For the fuel powder storage, it is found that a cylindrical tube container with a length of 500 [mm] and a diameter of 11 - 21 [mm] should be stored under water. For the fuel assembly assembling, CFD analysis indicates that the cladding tube temperature would satisfy the criterion if the inlet velocity of air is larger than 0.5 [m/s]. Through these studies, the new fuel composition which is consistent with the P&T cycle is obtained and the heat removal with the latest conditions is investigated. It is also shown that the new fuel composition can be practically handled with respect to heat generation, which is one of the most difficult points in handling MA fuel.
Koyama, Shinichi; Nakagiri, Toshio; Osaka, Masahiko; Yoshida, Hiroyuki; Kurata, Masaki; Ikeuchi, Hirotomo; Maeda, Koji; Sasaki, Shinji; Onishi, Takashi; Takano, Masahide; et al.
Hairo, Osensui Taisaku jigyo jimukyoku Homu Peji (Internet), 144 Pages, 2021/08
JAEA performed the subsidy program for the "Project of Decommissioning and Contaminated Water Management (Development of Analysis and Estimation Technology for Characterization of Fuel Debris (Development of Technologies for Enhanced Analysis Accuracy and Thermal Behavior Estimation of Fuel Debris))" in 2020JFY. This presentation summarized briefly the results of the project, which will be available shortly on the website of Management Office for the Project of Decommissioning and Contaminated Water Management.
Sakamoto, Atsushi; Kibe, Satoshi*; Kawanobe, Kazunori*; Fujisaku, Kazuhiko*; Sano, Yuichi; Takeuchi, Masayuki; Suzuki, Hideya*; Tsubata, Yasuhiro; Ban, Yasutoshi; Matsumura, Tatsuro
JAEA-Research 2021-003, 30 Pages, 2021/06
Japan Atomic Energy Agency has been developing a solvent extraction process called SELECT to recover minor actinides (MA) from spent nuclear fuel. In the SELECT process, TDdDGA, HONTA, and ADAAM are used as the extractants for MA + Ln corecovery, MA/Ln separation and Am/Cm separation, respectively. These extractants do not contain phosphorus (P), and consist of carbon (C), hydrogen (H), oxygen (O), and nitrogen (N). In this study, in order to give beneficial information for designing flowsheet, the mass transfer coefficients of Ln between HNO solution and TDdDGA or HONTA / n-dodecane solvent were evaluated by the single drop technique. Prior to the evaluation of mass transfer coefficient, we had optimized the structure of the single drop apparatus to improve accuracy of the measurement. Based on the mass transfer coefficients obtained in HNO
solution and TDdDGA or HONTA / n-dodecane solvent were evaluated by the single drop technique. Prior to the evaluation of mass transfer coefficient, we had optimized the structure of the single drop apparatus to improve accuracy of the measurement. Based on the mass transfer coefficients obtained in HNO / TDdDGA-n-dodecane system, Ln behaviors in the counter-current extraction and back-extraction using mixer-settlers and centrifugal contactors were estimated by simple calculation, and they had a good agreement with our previous experimental results. We also confirmed the mass transfer coefficients of Ln in HNO
/ TDdDGA-n-dodecane system, Ln behaviors in the counter-current extraction and back-extraction using mixer-settlers and centrifugal contactors were estimated by simple calculation, and they had a good agreement with our previous experimental results. We also confirmed the mass transfer coefficients of Ln in HNO / HONTA - n-dodecane system are under 10
/ HONTA - n-dodecane system are under 10 m/s.
m/s.
Toigawa, Tomohiro; Murayama, Rin*; Kumagai, Yuta; Yamashita, Shinichi*; Suzuki, Hideya; Ban, Yasutoshi; Matsumura, Tatsuro
UTNL-R-0501, p.24 - 25, 2020/12
This report summarizes the results obtained in FY2019 at Electron Linac Facility of University of Tokyo. The radiolysis process of a diglycolamide extractant, which is expected to be used in the separation process of minor actinides (MA), in dodecane and octanol solutions was investigated by pulse radiolysis. As a result, it was suggested that by adding alcohol, the decomposition process of the diglycolamide extractant was different from the decomposition processes in the single solvent of dodecane considered that the decomposition occurred via a radical cation species of the extractant.
Nakamura, Satoshi; Kimura, Takahiro; Ban, Yasutoshi; Tsubata, Yasuhiro; Matsumura, Tatsuro
JAEA-Technology 2020-009, 22 Pages, 2020/08
Partitioning and transmutation technology division is planning to measure fission rate ratios that contribute to validate nuclear data of minor actinides (MA). For this purpose, MA sources for fission chambers were prepared using electrodeposition method. The radioactivity of each MA source was quantified, and its uncertainty was evaluated. Seven types of MA sources with different radioactivity were prepared using four nuclides of  Np,
Np,  Am,
Am,  Am, and
Am, and  Cm. A
Cm. A  Cm source solution of which radioactivity was quantified by isotope dilution method was used to prepare working standard sources of
Cm source solution of which radioactivity was quantified by isotope dilution method was used to prepare working standard sources of  Cm. The radioactivities were quantified as 1461 Bq, 2179 Bq, and 2938 Bq for
Cm. The radioactivities were quantified as 1461 Bq, 2179 Bq, and 2938 Bq for  Np sources, 1.428 MBq for
Np sources, 1.428 MBq for  Am source, 370.5 kBq and 89.57 kBq for
Am source, 370.5 kBq and 89.57 kBq for  Am sources, and 2.327 MBq for
Am sources, and 2.327 MBq for  Cm source with, the uncertainty of 0.35% (1
Cm source with, the uncertainty of 0.35% (1 ). This report summarizes the method for preparation and quantification of MA sources, and uncertainty evaluation.
). This report summarizes the method for preparation and quantification of MA sources, and uncertainty evaluation.
Tsutsui, Nao; Ban, Yasutoshi; Suzuki, Hideya*; Nakase, Masahiko*; Ito, Sayumi*; Inaba, Yusuke*; Matsumura, Tatsuro; Takeshita, Kenji*
Analytical Sciences, 36(2), p.241 - 246, 2020/02
Times Cited Count:30 Percentile:83.01(Chemistry, Analytical)To investigate the effective separation of actinides (Ans) from lanthanides (Lns), single-stage batch extraction experiments were performed with a novel extractant, tetradodecyl-1,10-phenanthroline-2,9-diamide (TDdPTDA) with various diluents such as 3-nitrobenzotrifluoride (F-3), nitrobenzene, and  -dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from
-dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from  -dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
-dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
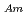 ) and Cm (
) and Cm (
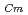 ) for TDdPTDA, with the high separation factors (
) for TDdPTDA, with the high separation factors ( s) of Am from Lns enough for their separation.
s) of Am from Lns enough for their separation.
Sasaki, Yuji; Ban, Yasutoshi; Morita, Keisuke; Matsumiya, Masahiko*; Ono, Ryoma*; Shiroishi, Hidenobu*
Solvent Extraction Research and Development, Japan, 27(1), p.63 - 67, 2020/00
Times Cited Count:6 Percentile:23.87(Chemistry, Multidisciplinary)Mutual separation technique of Dy and Nd in Nd magnet is studied. Dy is more valuable than Nd, then Dy might be isolated and reused. Lanthanide elements can be extracted thoroughly by diglycolamide (DGA) extractants, we use this reagent for the recovery and isolation of Dy. Tetradodecyl-DGA (TDdDGA) has relatively high separation factors(SF) between Dy and Nd (SF=17-18) in HNO extraction system, counter-current extraction using TDdDGA was applied for their mutual separation. From the present study, using the condition, four extraction stages, organic phase: 0.1M TDdDGA in n-dodecane, aqueous phase: 0.3M HNO
extraction system, counter-current extraction using TDdDGA was applied for their mutual separation. From the present study, using the condition, four extraction stages, organic phase: 0.1M TDdDGA in n-dodecane, aqueous phase: 0.3M HNO , 92% Dy can be recovered with 0.7% co-extraction of Nd.
, 92% Dy can be recovered with 0.7% co-extraction of Nd.