Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Johansen, M. P.*; Child, D. P.*; Collins, R.*; Cook, M.*; Davis, J.*; Hotchkis, M. A. C.*; Howard, D. L.*; Howell, N.*; Ikeda, Atsushi; Young, E.*
Science of the Total Environment, 842, p.156755_1 - 156755_11, 2022/10
Times Cited Count:7 Percentile:39.89(Environmental Sciences) -octylnitrilo-triacetamide (HONTA) complexes of americium and europium
-octylnitrilo-triacetamide (HONTA) complexes of americium and europiumToigawa, Tomohiro; Peterman, D. R.*; Meeker, D. S.*; Grimes, T. S.*; Zalupski, P. R.*; Mezyk, S. P.*; Cook, A. R.*; Yamashita, Shinichi*; Kumagai, Yuta; Matsumura, Tatsuro; et al.
Physical Chemistry Chemical Physics, 23(2), p.1343 - 1351, 2021/01
Times Cited Count:21 Percentile:83.18(Chemistry, Physical)The candidate An(III)/Ln(III) separation ligand hexa- -octylnitrilo-triacetamide (HONTA) was irradiated under envisioned SELECT (Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation) process conditions using a solvent test loop in conjunction with cobalt-60 gamma irradiation. We demonstrate that HONTA undergoes exponential decay with increasing gamma dose to produce a range of degradation products which have been identified and quantified by HPLC-ESI-MS/MS techniques. The combination of HONTA destruction and degradation product ingrowth, particularly dioctylamine, negatively impacts the extraction and back-extraction of both americium and europium ions. The loss of HONTA was attributed to its reaction with the solvent (
-octylnitrilo-triacetamide (HONTA) was irradiated under envisioned SELECT (Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation) process conditions using a solvent test loop in conjunction with cobalt-60 gamma irradiation. We demonstrate that HONTA undergoes exponential decay with increasing gamma dose to produce a range of degradation products which have been identified and quantified by HPLC-ESI-MS/MS techniques. The combination of HONTA destruction and degradation product ingrowth, particularly dioctylamine, negatively impacts the extraction and back-extraction of both americium and europium ions. The loss of HONTA was attributed to its reaction with the solvent ( -dodecane) radical cation of
-dodecane) radical cation of 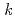 (HONTA + R
(HONTA + R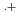 ) = (7.61
) = (7.61  0.82)
0.82)  10
10 M
M s
s obtained by pulse radiolysis techniques. However, when this ligand is bound to either americium or europium ions, the observed
obtained by pulse radiolysis techniques. However, when this ligand is bound to either americium or europium ions, the observed  -dodecane radical cation kinetics increase by over an order of magnitude. This large reactivity increase to additional reaction pathways occurring upon metal-ion binding. Lastly nanosecond time-resolved measurements showed that both direct and indirect HONTA radiolysis yielded the short-lived (
-dodecane radical cation kinetics increase by over an order of magnitude. This large reactivity increase to additional reaction pathways occurring upon metal-ion binding. Lastly nanosecond time-resolved measurements showed that both direct and indirect HONTA radiolysis yielded the short-lived ( 100 ns) HONTA radical cation as well as a longer-lived (
100 ns) HONTA radical cation as well as a longer-lived ( s) HONTA triplet excited state. These HONTA species are important precursors to the suite of HONTA degradation products observed.
s) HONTA triplet excited state. These HONTA species are important precursors to the suite of HONTA degradation products observed.
 -ray spectroscopy of
-ray spectroscopy of 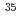 Mg and
Mg and 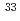 Na
NaGade, A.*; Basin, D.*; Brown, B. A.*; Campbell, C. M.*; Cook, J. M.*; Ettenauer, S.*; Glasmacher, T.*; Kemper, K. W.*; McDaniel, S.*; Obertelli, A.*; et al.
Physical Review C, 83(4), p.044305_1 - 044305_5, 2011/04
Times Cited Count:22 Percentile:74.39(Physics, Nuclear)no abstracts in English
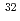 Mg; Intruder amplitudes in
Mg; Intruder amplitudes in 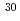 Ne and implications for the binding of
Ne and implications for the binding of 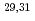 F
FFallon, P.*; Rodriguez-Vieitez, E.*; Macchiavelli, A. O.*; Gade, A.*; Tostevin, J. A.*; Adrich, P.*; Bazin, D.*; Bowen, M.*; Campbell, C. M.*; Clark, R. M.*; et al.
Physical Review C, 81(4), p.041302_1 - 041302_5, 2010/04
Times Cited Count:42 Percentile:88.34(Physics, Nuclear)no abstracts in English
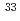 Mg and
Mg and 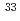 Al
AlTripathi, V.*; Tabor, S. L.*; Mantica, P. F.*; Utsuno, Yutaka; Bender, P.*; Cook, J.*; Hoffman, C. R.*; Lee, S.*; Otsuka, Takaharu*; Pereira, J.*; et al.
Physical Review Letters, 101(14), p.142504_1 - 142504_4, 2008/10
Times Cited Count:57 Percentile:86.73(Physics, Multidisciplinary)no abstracts in English
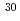 Na
NaEttenauer, S.*; Zwahlen, H.*; Adrich, P.*; Bazin, D.*; Campbell, C. M.*; Cook, J. M.*; Davies, A. D.*; Dinca, D.-C.*; Gade, A.*; Glasmacher, T.*; et al.
Physical Review C, 78(1), p.017302_1 - 017302_4, 2008/07
Times Cited Count:14 Percentile:63.86(Physics, Nuclear)no abstracts in English
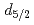 proton hole strength in neutron-rich
proton hole strength in neutron-rich 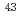 P; Shell structure and nuclear shapes near
P; Shell structure and nuclear shapes near  =28
=28Riley, L. A.*; Adrich, P.*; Baugher, T. R.*; Bazin, D.*; Brown, B. A.*; Cook, J. M.*; Cottle, P. D.*; Diget, C. A.*; Gade, A.*; Garland, D. A.*; et al.
Physical Review C, 78(1), p.011303_1 - 011303_5, 2008/07
Times Cited Count:25 Percentile:78.21(Physics, Nuclear)no abstracts in English
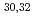 Mg; Mapping the transition into the "island of inversion"
Mg; Mapping the transition into the "island of inversion"Terry, J. R.*; Brown, B. A.*; Campbell, C. M.*; Cook, J. M.*; Davies, A. D.*; Dinca, D.-C.*; Gade, A.*; Glasmacher, T.*; Hansen, P. G.*; Sherrill, B. M.*; et al.
Physical Review C, 77(1), p.014316_1 - 014316_12, 2008/01
Times Cited Count:79 Percentile:94.95(Physics, Nuclear)no abstracts in English
Tripathi, V.*; Tabor, S. L.*; Mantica, P. F.*; Utsuno, Yutaka; Bender, P.*; Cook, J. M.*; Hoffman, C. R.*; Lee, S.*; Otsuka, Takaharu*; Pereira, J.*; et al.
Physical Review C, 76(2), p.021301_1 - 021301_5, 2007/08
Times Cited Count:28 Percentile:82.56(Physics, Nuclear)no abstracts in English
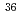 Mg; Interplay of normal and intruder configurations at the neutron-rich boundary of the "Island of Inversion"
Mg; Interplay of normal and intruder configurations at the neutron-rich boundary of the "Island of Inversion"Gade, A.*; Adrich, P.*; Bazin, D.*; Bowen, M. D.*; Brown, B. A.*; Campbell, C. M.*; Cook, J. M.*; Ettenauer, S.*; Glasmacher, T.*; Kemper, K. W.*; et al.
Physical Review Letters, 99(7), p.072502_1 - 072502_4, 2007/08
Times Cited Count:78 Percentile:90.10(Physics, Multidisciplinary)no abstracts in English
 I/
I/ I ratios in surface waters of the English Lake District
I ratios in surface waters of the English Lake DistrictAtarashi-Andoh, Mariko; Schnabel, C.*; Cook, G.*; MacKenzie, A. B.*; Dougans, A.*; Ellam, R. M.*; Freeman, S.*; Maden, C.*; Olive, V.*; Synal, H.-A.*; et al.
Applied Geochemistry, 22(3), p.628 - 636, 2007/03
Times Cited Count:16 Percentile:37.87(Geochemistry & Geophysics)Accelerator Mass Spectrometry (AMS) was used to measure  I/
I/ I ratios in surface sea, lake and river water samples collected in 2004 and 2005 from the English Lake District and from South west Scotland, areas which are in relatively close proximity to the Sellafield nuclear fuel reprocessing plant in north west England. The
I ratios in surface sea, lake and river water samples collected in 2004 and 2005 from the English Lake District and from South west Scotland, areas which are in relatively close proximity to the Sellafield nuclear fuel reprocessing plant in north west England. The  I/
I/ I ratios in surface water collected from the shore of the Irish Sea were in the range 2.8
I ratios in surface water collected from the shore of the Irish Sea were in the range 2.8 10
10 to 8.2
to 8.2 10
10 . These ratios are one order of magnitude higher than that of seawater collected from the Irish Sea in 1992, correlating with the increase in
. These ratios are one order of magnitude higher than that of seawater collected from the Irish Sea in 1992, correlating with the increase in  I content of the Sellafield liquid effluent discharge over the last decade. The
I content of the Sellafield liquid effluent discharge over the last decade. The  I/
I/ I ratios in lakes in the Lake District were in the range 0.7
I ratios in lakes in the Lake District were in the range 0.7 10
10 to 6.4
to 6.4 10
10 and decreased exponentially as a function of distance from Sellafield.
and decreased exponentially as a function of distance from Sellafield.
Terry, J. R.*; Basin, D.*; Brown, B. A.*; Campbell, C. M.*; Church, J. A.*; Cook, J. M.*; Davies, A. D.*; Dinca, D.-C.*; Enders, J.*; Gade, A.*; et al.
Physics Letters B, 640(3), p.86 - 90, 2006/09
Times Cited Count:60 Percentile:92.88(Astronomy & Astrophysics)no abstracts in English