Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Schyman, P.*; Danielsson, J.*; Pinak, M.; Laaksonen, A.*
Journal of Physical Chemistry A, 109(8), p.1713 - 1719, 2005/02
Times Cited Count:39 Percentile:77.29(Chemistry, Physical)We have examined the role of the catalytic lysine (Lys 249) in breaking the glycosidic bond of 8-oxoguanine in the enzyme human 8-oxoguanine DNA glycosylase. It has been assumed that this lysine acts as a nucleophile in a S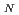 2 type of reaction after being activated through a donation of a proton to a strictly conserved aspartate. We use hybrid density functional theory to characterize both associative and dissociative pathways. We find that the smallest energetical barrier involves a S
2 type of reaction after being activated through a donation of a proton to a strictly conserved aspartate. We use hybrid density functional theory to characterize both associative and dissociative pathways. We find that the smallest energetical barrier involves a S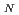 1 type of mechanism where the lysine electrostatically stabilizes the dissociating base and then donates a proton with a very small barrier and then finally attacks the sugar ring to create the covalently bounded protein-DNA intermediate complex. Reported findings give further support to the assumption that a dissociative mechanism may be the preferred mode of action for this type of enzymes.
1 type of mechanism where the lysine electrostatically stabilizes the dissociating base and then donates a proton with a very small barrier and then finally attacks the sugar ring to create the covalently bounded protein-DNA intermediate complex. Reported findings give further support to the assumption that a dissociative mechanism may be the preferred mode of action for this type of enzymes.