Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 TiO
TiO during DT neutron irradiation by use of an improved tritium collection method
during DT neutron irradiation by use of an improved tritium collection methodEdao, Yuki; Kawamura, Yoshinori; Hoshino, Tsuyoshi; Ochiai, Kentaro
Fusion Engineering and Design, 112, p.480 - 485, 2016/11
Times Cited Count:8 Percentile:55.65(Nuclear Science & Technology)The accurate measurement of behavior of bred tritium released from a tritium breeder is indispensable to understand the behavior for a design of a tritium extraction system. The tritium collection method combined a CuO bed and water bubbles was not suitable to measure transient behavior of tritium released from Li TiO
TiO during neutron irradiation because tritium released behavior was changed to be delayed due to adsorption of oxidized tritium on the CuO. Hence, the tritium collection method with hydrophobic catalyst instead of the CuO was demonstrated and succeeded the accurate release measurement of tritium from Li
during neutron irradiation because tritium released behavior was changed to be delayed due to adsorption of oxidized tritium on the CuO. Hence, the tritium collection method with hydrophobic catalyst instead of the CuO was demonstrated and succeeded the accurate release measurement of tritium from Li TiO
TiO . With the method, we assessed the behavior of tritium release under the various conditions since tritium should be released from Li
. With the method, we assessed the behavior of tritium release under the various conditions since tritium should be released from Li TiO
TiO as the form of HT as much as possible from the view point of the fuel cycle. Our results indicated; promotion of isotopic exchange reaction on the surface of Li
as the form of HT as much as possible from the view point of the fuel cycle. Our results indicated; promotion of isotopic exchange reaction on the surface of Li TiO
TiO by addition of hydrogen in sweep gas is mandatory in order to release tritium smoothly from Li
by addition of hydrogen in sweep gas is mandatory in order to release tritium smoothly from Li TiO
TiO irradiated with neutrons; the favorable sweep gas to release as the form of HT was hydrogen added inert gas; and the temperature of Li
irradiated with neutrons; the favorable sweep gas to release as the form of HT was hydrogen added inert gas; and the temperature of Li TiO
TiO was the dominant parameter to control the chemical form of tritium released from the Li
was the dominant parameter to control the chemical form of tritium released from the Li TiO
TiO .
.
Edao, Yuki; Sato, Katsumi; Iwai, Yasunori; Hayashi, Takumi
Journal of Nuclear Science and Technology, 53(11), p.1831 - 1838, 2016/11
Times Cited Count:9 Percentile:59.71(Nuclear Science & Technology)Edao, Yuki; Iwai, Yasunori; Sato, Katsumi; Hayashi, Takumi
Applied Radiation and Isotopes, 114, p.40 - 44, 2016/08
Times Cited Count:2 Percentile:17.75(Chemistry, Inorganic & Nuclear)We have focused on bacterial oxidation of tritium by hydrogen-oxidizing bacteria in natural soil to realize a passive reactor for tritium oxidation at room temperature. The purpose of this study was to examine the feasibility of a bioreactor with hydrogen-oxidizing bacteria for detritiation system from a point of view of engineering. The efficiency of the bioreactor was evaluated by kinetics. The bioreactor packed with natural soil shows a relative high conversion rate of tritium under the saturated moisture condition at room temperature, which is obviously superior to that of a Pt/Al O
O catalyst generally used for tritium oxidation in the existing tritium handling facilities. The order of reaction for tritium oxidation with soil was the pseudo-first order as assessed with Michaelis-Menten kinetics model. Our engineering suggestion to increase the reaction rate is the intentional addition of hydrogen at a small concentration in the feed gas on condition that the oxidation of tritium with soil is expressed by the Michaelis-Menten kinetics model. We reach a conclusion that a bioreactor using hydrogen-oxidizing bacteria in natural soil has the feasibility to be applied in the DS as a passive reactor.
catalyst generally used for tritium oxidation in the existing tritium handling facilities. The order of reaction for tritium oxidation with soil was the pseudo-first order as assessed with Michaelis-Menten kinetics model. Our engineering suggestion to increase the reaction rate is the intentional addition of hydrogen at a small concentration in the feed gas on condition that the oxidation of tritium with soil is expressed by the Michaelis-Menten kinetics model. We reach a conclusion that a bioreactor using hydrogen-oxidizing bacteria in natural soil has the feasibility to be applied in the DS as a passive reactor.
Isobe, Kanetsugu; Kawamura, Yoshinori; Iwai, Yasunori; Oyaizu, Makoto; Nakamura, Hirofumi; Suzuki, Takumi; Yamada, Masayuki; Edao, Yuki; Kurata, Rie; Hayashi, Takumi; et al.
Fusion Engineering and Design, 98-99, p.1792 - 1795, 2015/10
Times Cited Count:0 Percentile:0.00(Nuclear Science & Technology)Activities on Broader Approach (BA) were started in 2007 on the basis of the Agreement between the Government of Japan and the EURATOM. The period of BA activities consist of Phase1 and Phase2 dividing into Phase 2-1 (2010-2011), Phase 2-2 (2012-2013) and Phase 2-3 (2014-2016). Tritium technology was chosen as one of important R&D issues to develop DEMO plant. R&D activities of tritium technology on BA consist of four tasks. Task-1 is to prepare and maintain the tritium handling facility in Rokkasho BA site in Japan. Task 2, 3 and 4 are main R&D activities for tritium and these are focused on: Task-2) Development of tritium accountancy technology, Task-3) Development of basic tritium safety research, Task-4) Tritium durability test. R&D activities of tritium technology in Phase 2-2 were underway successfully and closed in 2013.
Iwai, Yasunori; Kubo, Hitoshi*; Oshima, Yusuke*; Noguchi, Hiroshi*; Edao, Yuki; Taniuchi, Junichi*
Fusion Science and Technology, 68(3), p.596 - 600, 2015/10
Times Cited Count:2 Percentile:16.04(Nuclear Science & Technology)We have newly developed the hydrophobic platinum honeycomb catalysts applicable to tritium oxidation reactor since the honeycomb-shape catalyst can decrease the pressure drop. Two types of hydrophobic honeycomb catalyst have been test-manufactured. One is the hydrophobic platinum catalyst on a metal honeycomb. The other is the hydrophobic platinum catalyst on a ceramic honeycomb made of silicon carbide. The fine platinum particles around a few nanometers significantly improve the catalytic activity for the oxidation tritium at a tracer concentration. The hydrogen concentration in the gaseous feed slightly affects the overall reaction rate constant for hydrogen oxidation. Due to the competitive adsorption of hydrogen and water molecules on platinum surface, the overall reaction rate constant has the bottom value. The hydrogen concentration for the bottom value is 100 ppm under the dry feed gas. We have experimentally confirmed the activity of these honeycomb catalysts is as good as that of pellet-shape hydrophobic catalyst. The results support the hydrophobic honeycomb catalysts are applicable to tritium oxidation reactor.
Hayashi, Takumi; Nakamura, Hirofumi; Kawamura, Yoshinori; Iwai, Yasunori; Isobe, Kanetsugu; Yamada, Masayuki; Suzuki, Takumi; Kurata, Rie; Oyaizu, Makoto; Edao, Yuki; et al.
Fusion Science and Technology, 67(2), p.365 - 370, 2015/03
Times Cited Count:1 Percentile:8.75(Nuclear Science & Technology)Edao, Yuki; Kawamura, Yoshinori; Kurata, Rie; Fukada, Satoshi*; Takeishi, Toshiharu*; Hayashi, Takumi; Yamanishi, Toshihiko
Fusion Science and Technology, 67(2), p.320 - 323, 2015/03
Times Cited Count:0 Percentile:0.00(Nuclear Science & Technology)The present study aims at obtaining fundamental knowledge for tritium transfer behavior and interaction between tritium and paint coated on concrete walls. The amounts of tritium penetration and release in cement paste with epoxy and urethane paint coatings were measured. The tritium penetration amounts were increased with the HTO exposure time. Time to achieve each saturate tritium value was more than 60 days for cement paste coated with epoxy paint and with urethane paint, while cement paste without paint took 2 days to achieve it. Tritium penetration rates were estimated by an analysis of diffusion model. Although their paint coatings were effective for reduction of tritium penetration through the cement paste exposed to HTO for a short period, the amount of tritium trapped in the paints became large for a long time. This work has been performed under the collaboration research between JAEA and Kyushu University.
Fukada, Satoshi*; Katayama, Kazunari*; Takeishi, Toshiharu*; Edao, Yuki; Kawamura, Yoshinori; Hayashi, Takumi; Yamanishi, Toshihiko
Fusion Science and Technology, 67(2), p.99 - 102, 2015/03
Times Cited Count:0 Percentile:0.00(Nuclear Science & Technology)Hoshino, Tsuyoshi; Ochiai, Kentaro; Edao, Yuki; Kawamura, Yoshinori
Fusion Science and Technology, 67(2), p.386 - 389, 2015/03
Times Cited Count:15 Percentile:73.95(Nuclear Science & Technology)Demonstration power reactors (DEMOs) require advanced tritium breeders that have high stability at high temperatures. Therefore, the pebble fabrication of Li TiO
TiO with excess Li (Li
with excess Li (Li TiO
TiO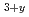 ) as an advanced tritium breeder was carried out. In this study, a preliminary examination of the tritium release properties of advanced tritium breeders was performed. DT neutron irradiation experiments were performed at the fusion neutronics source (FNS) facility in JAEA. The Li
) as an advanced tritium breeder was carried out. In this study, a preliminary examination of the tritium release properties of advanced tritium breeders was performed. DT neutron irradiation experiments were performed at the fusion neutronics source (FNS) facility in JAEA. The Li TiO
TiO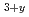 pebbles exhibited good tritium release properties similar to the Li
pebbles exhibited good tritium release properties similar to the Li TiO
TiO pebbles. In particular, the released amount of HT gas for easier tritium handling was higher than that of HTO water.
pebbles. In particular, the released amount of HT gas for easier tritium handling was higher than that of HTO water.
Kawamura, Yoshinori; Edao, Yuki; Iwai, Yasunori; Hayashi, Takumi; Yamanishi, Toshihiko
Fusion Engineering and Design, 89(7-8), p.1539 - 1543, 2014/10
Times Cited Count:9 Percentile:49.93(Nuclear Science & Technology)Tritium recovery system using adsorption or catalytic isotope exchange has already been proposed for a solid breeding blanket system of a nuclear fusion reactor. Synthetic zeolite is often used as an adsorbent or a substrate of chemical exchange catalyst. And, it is well known that its properties are changed easily by exchanging their cations. So, in this work, adsorption capacities of hydrogen isotope and water vapor on cation-exchanged mordenite with transition metal ion were investigated. Ag ion-exchanged mordenite (Ag-MOR) has indicated considerably large hydrogen adsorption capacity in lower pressure range at 77 K. And, adsorption capacity of water vapor did not so vary with exchaned cation in comparison with hydrogen adsorption. The discussion from the viewpoint of adsorption rate is still remaining, but more compact cryosorption column for tritium recovery system is possible to design if Ag-MOR is adopted.
Edao, Yuki; Kawamura, Yoshinori; Yamanishi, Toshihiko; Fukada, Satoshi*
Fusion Engineering and Design, 89(9-10), p.2062 - 2065, 2014/10
Times Cited Count:4 Percentile:28.36(Nuclear Science & Technology)Tritium transfer behavior through hydrophobic paints, epoxy resin and acrylic-silicon resin, was investigated experimentally. The authors measured the amount of tritium permeated through the paint membranes which exposed in HTO atmosphere of 2 100 Bq/cm
100 Bq/cm . The most of tritium permeated through the paints in the form of HTO at room temperature. Tritium permeation through the acrylic-silicon paint was explained a linear sorption/release model and that through the epoxy paint was suggested to be controlled by a one-dimensional diffusion model. While effective diffusivity was 1.0
. The most of tritium permeated through the paints in the form of HTO at room temperature. Tritium permeation through the acrylic-silicon paint was explained a linear sorption/release model and that through the epoxy paint was suggested to be controlled by a one-dimensional diffusion model. While effective diffusivity was 1.0 10
10
 1.8
1.8 10
10 m
m /s at 21
/s at 21 C
C 26
26 C for epoxy membrane, the diffusivity was found to be hundreds times larger than that for cement-paste coated with epoxy paint. Hence, tritium diffusivity through interface between cement-paste and the epoxy paint was considered to be most effective in the overall tritium transfer process. Tritium transfer behavior in the interface is important to explain the mechanism of tritium transfer behavior in concrete walls.
C for epoxy membrane, the diffusivity was found to be hundreds times larger than that for cement-paste coated with epoxy paint. Hence, tritium diffusivity through interface between cement-paste and the epoxy paint was considered to be most effective in the overall tritium transfer process. Tritium transfer behavior in the interface is important to explain the mechanism of tritium transfer behavior in concrete walls.
Ochiai, Kentaro; Kawamura, Yoshinori; Hoshino, Tsuyoshi; Edao, Yuki; Takakura, Kosuke; Ota, Masayuki; Sato, Satoshi; Konno, Chikara
Fusion Engineering and Design, 89(7-8), p.1464 - 1468, 2014/10
Times Cited Count:9 Percentile:53.95(Nuclear Science & Technology)We have performed the tritium recovery experiment on fusion reactor blanket with DT neutrons at the Fusion Neutronics Source facility in Japan Atomic Energy Agency. The candidate breeding material, Li TiO
TiO pebble, was put into the container which was set up it into an assembly simulating water cooled ceramic breeding (WCCB) blanket. Helium sweep gas including H
pebble, was put into the container which was set up it into an assembly simulating water cooled ceramic breeding (WCCB) blanket. Helium sweep gas including H (1%) and/or H
(1%) and/or H O (1%) was flowed and extracted tritium was collected to water bubblers during DT neutron irradiation. The Li
O (1%) was flowed and extracted tritium was collected to water bubblers during DT neutron irradiation. The Li TiO
TiO pebble was also heated up to a constant temperature at 573, 873 and 1073 K, respectively. We arranged the tritium recovery system to measure tritiated water moisture and tritium gas, separately, and to investigate the amount of recovered tritium and the chemical form. From our experiments, it was showed that the amount of recovered tritium was corresponded to the calculation value and the ratio of chemical form depended to the temperature and kinds of sweep gas.
pebble was also heated up to a constant temperature at 573, 873 and 1073 K, respectively. We arranged the tritium recovery system to measure tritiated water moisture and tritium gas, separately, and to investigate the amount of recovered tritium and the chemical form. From our experiments, it was showed that the amount of recovered tritium was corresponded to the calculation value and the ratio of chemical form depended to the temperature and kinds of sweep gas.
Hayashi, Takumi; Isobe, Kanetsugu; Nakamura, Hirofumi; Kobayashi, Kazuhiro; Oya, Yasuhisa*; Okuno, Kenji*; Oyaizu, Makoto; Edao, Yuki; Yamanishi, Toshihiko
Fusion Engineering and Design, 89(7-8), p.1520 - 1523, 2014/10
Times Cited Count:3 Percentile:21.88(Nuclear Science & Technology)Tritium confinement is the most important safety issue in the fusion reactor. Tritium behavior on the water metal boundary is very important to design tritium plant with breading blanket system using cooling water. A series of tritium permeation experiment into pressurized water or water vapor jacket with He or Ar have been performed through pure iron piping with/without 7 micro-meter gold plating, which contained about 1 kPa of pure tritium gas at 423 K, with monitoring the chemical forms of tritium. Also, deuterium permeation experiments from heavy water vessel through various metal piping, such as pure iron (Fe), nickel (Ni), stainless steel (SS304), and pure iron with 10 micro-meter gold plating, were performed at 573 K and at 15 MPa. Recently, using the above heavy water system, we have succeeded to detect simultaneous hydrogen isotopes transfer from and to the metal surface by introducing H gas to the metal piping after stabilized deuterium permeation was detected.
gas to the metal piping after stabilized deuterium permeation was detected.
Wakai, Eiichi; Kondo, Hiroo; Kanemura, Takuji; Furukawa, Tomohiro; Hirakawa, Yasushi; Watanabe, Kazuyoshi; Ida, Mizuho*; Ito, Yuzuru; Niitsuma, Shigeto; Edao, Yuki; et al.
Fusion Science and Technology, 66(1), p.46 - 56, 2014/07
Times Cited Count:4 Percentile:28.36(Nuclear Science & Technology)Kawamura, Yoshinori; Edao, Yuki; Yamanishi, Toshihiko
Fusion Engineering and Design, 88(9-10), p.2255 - 2258, 2013/10
Times Cited Count:10 Percentile:58.46(Nuclear Science & Technology)To develop an adsorbent that is suitable for a separation column of gas chromatograph for hydrogen isotope analysis, the mordenite-type zeolite of which cations (Na ) were exchanged with other cations have been prepared and their hydrogen isotope adsorption behavior is being investigated. Then, it has been shown experimentally that mordenite-type zeolite of which cation has been exchanged with Ca
) were exchanged with other cations have been prepared and their hydrogen isotope adsorption behavior is being investigated. Then, it has been shown experimentally that mordenite-type zeolite of which cation has been exchanged with Ca (Ca-MOR) has fairly large adsorption capacity. So, breakthrough curves of H
(Ca-MOR) has fairly large adsorption capacity. So, breakthrough curves of H (or D
(or D ) adsorption on Ca-MOR at 194 K and 175 K have been observed and mass transfer coefficients have been estimated from them. The rate-controlling step of hydrogen adsorption is hydrogen diffusion in porous adsorbent. And, isotopic difference of effective diffusivity in Ca-MOR is larger than that in Na-MOR. Therefore, in comparison with Na-MOR, use of Ca-MOR is expected to enhance the hydrogen isotope separation capability.
) adsorption on Ca-MOR at 194 K and 175 K have been observed and mass transfer coefficients have been estimated from them. The rate-controlling step of hydrogen adsorption is hydrogen diffusion in porous adsorbent. And, isotopic difference of effective diffusivity in Ca-MOR is larger than that in Na-MOR. Therefore, in comparison with Na-MOR, use of Ca-MOR is expected to enhance the hydrogen isotope separation capability.
 TiO
TiO under D-T neutron irradiation
under D-T neutron irradiationEdao, Yuki; Kawamura, Yoshinori; Ochiai, Kentaro; Hoshino, Tsuyoshi; Takakura, Kosuke; Ota, Masayuki; Iwai, Yasunori; Yamanishi, Toshihiko; Konno, Chikara
JAEA-Research 2012-040, 15 Pages, 2013/02
Tritium generation and recovery studies on Li TiO
TiO as a solid breeding material under neutron irradiation carried out in the Fusion Neutron Source (FNS) facility. A capsule with Li
as a solid breeding material under neutron irradiation carried out in the Fusion Neutron Source (FNS) facility. A capsule with Li TiO
TiO packed bed was put in a system which simulated an actual blanket system which built in beryllium blocks and lithium titanate ones. Estimated values of the amount of tritium generation by a numerical calculation agreed closely with experimental values. The capsule was heated up to 300
packed bed was put in a system which simulated an actual blanket system which built in beryllium blocks and lithium titanate ones. Estimated values of the amount of tritium generation by a numerical calculation agreed closely with experimental values. The capsule was heated up to 300 C, and helium, helium with water vapor, hydrogen or hydrogen/water vapor were selected as purge gas. In the case of purge by helium added water vapor, the ratio of HTO to total tritium release was 98%. In helium with hydrogen/water vapor purge, the ratio of HTO to total tritium release was 80%, which was confirmed that HTO released by isotope exchange reaction between water vapor and tritium. In helium with hydrogen purge, the ratio of HT to total tritium release was 60
C, and helium, helium with water vapor, hydrogen or hydrogen/water vapor were selected as purge gas. In the case of purge by helium added water vapor, the ratio of HTO to total tritium release was 98%. In helium with hydrogen/water vapor purge, the ratio of HTO to total tritium release was 80%, which was confirmed that HTO released by isotope exchange reaction between water vapor and tritium. In helium with hydrogen purge, the ratio of HT to total tritium release was 60 70%, which was shown that HT released by isotope exchange reaction between hydrogen gas and tritium. HTO released by water generation reaction between hydrogen in purge gas and oxygen in Li
70%, which was shown that HT released by isotope exchange reaction between hydrogen gas and tritium. HTO released by water generation reaction between hydrogen in purge gas and oxygen in Li TiO
TiO although water vapor was not added in purge gas. The ratio of HTO release seemed to be small under the deoxidized condition of the Li
although water vapor was not added in purge gas. The ratio of HTO release seemed to be small under the deoxidized condition of the Li TiO
TiO surface. Tritium release behavior in the Li
surface. Tritium release behavior in the Li TiO
TiO depended on the composition of purge gas, and its chemical form was affected by the surface conditions of Li
depended on the composition of purge gas, and its chemical form was affected by the surface conditions of Li TiO
TiO .
.
Wakai, Eiichi; Kondo, Hiroo; Sugimoto, Masayoshi; Fukada, Satoshi*; Yagi, Juro*; Ida, Mizuho; Kanemura, Takuji; Furukawa, Tomohiro; Hirakawa, Yasushi; Watanabe, Kazuyoshi; et al.
Purazuma, Kaku Yugo Gakkai-Shi, 88(12), p.691 - 705, 2012/12
no abstracts in English
Fukada, Satoshi*; Edao, Yuki*; Sato, Koichi*; Takeishi, Toshiharu*; Katayama, Kazunari*; Kobayashi, Kazuhiro; Hayashi, Takumi; Yamanishi, Toshihiko; Hatano, Yuji*; Taguchi, Akira*; et al.
Fusion Engineering and Design, 87(1), p.54 - 60, 2012/01
Times Cited Count:4 Percentile:30.25(Nuclear Science & Technology)An experimental study on tritium (T) transfer in porous concrete for the tertiary T safety containment is performed to investigate (1) how fast HTO penetrates through concrete walls, (2) how well concrete walls contaminated with water-soluble T are decontaminated by a solution-in-water technique, and (3) how well hydrophobic paint coating works as a protecting film against HTO migrating through concrete walls. The epoxy paint coating can work as a HTO diffusion barrier and the PRF value is around 1/10. The silicon paint coating cannot work as the anti-T permeation barrier, because water deteriorates contact between the paint and cement or mortar.
Nakamura, Kazuyuki; Furukawa, Tomohiro; Hirakawa, Yasushi; Kanemura, Takuji; Kondo, Hiroo; Ida, Mizuho; Niitsuma, Shigeto; Otaka, Masahiko; Watanabe, Kazuyoshi; Horiike, Hiroshi*; et al.
Fusion Engineering and Design, 86(9-11), p.2491 - 2494, 2011/10
Times Cited Count:11 Percentile:58.47(Nuclear Science & Technology)In IFMIF/EVEDA, tasks for lithium target system are shared to 5 validation tasks (LF1-5) and a design task (LF6). The purpose of LF1 task is to construct and operate the EVEDA lithium test loop, and JAEA has a main responsibility to the performance of the Li test loop. LF2 is a task for the diagnostics of the Li test loop and IFMIF design. Basic research for the diagnostics equipment has been completed, and the construction for the Li test loop will be finished before March in 2011. LF4 is a task for the purification systems with nitrogen and hydrogen. Basic research for the purification equipment has been completed, and the construction of the nitrogen system for the Li test loop will be finished before March in 2011. LF5 is a task for the remote handling system with the target assembly. JAEA has an idea to use the laser beam for cutting and welding of the lip part of the flanges. LF6 is a task for the design of the IFMIF based on the validation experiments of LF1-5.
Kondo, Hiroo; Furukawa, Tomohiro; Hirakawa, Yasushi; Nakamura, Hiroo*; Ida, Mizuho; Watanabe, Kazuyoshi; Miyashita, Makoto*; Horiike, Hiroshi*; Yamaoka, Nobuo*; Kanemura, Takuji; et al.
Proceedings of 23rd IAEA Fusion Energy Conference (FEC 2010) (CD-ROM), 8 Pages, 2010/10
The Engineering Validation and Engineering Design Activity (EVEDA) for the International Fusion Materials Irradiation Facility (IFMIF) is proceeded as one of the ITER Broader Approach (BA) activities. The EVEDA Li test loop (ELTL) is aimed at validating stability of the Li target and feasibility of a Li purification system as the key issues. In this paper, the design of the ELTL especially of a target assembly in which the Li target is produced by the contraction nozzle is presented.