Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sato, Tetsuya; Asai, Masato; Borschevsky, A.*; Beerwerth, R.*; Kaneya, Yusuke*; Makii, Hiroyuki; Mitsukai, Akina*; Nagame, Yuichiro; Osa, Akihiko; Toyoshima, Atsushi; et al.
Journal of the American Chemical Society, 140(44), p.14609 - 14613, 2018/11
Times Cited Count:32 Percentile:69.40(Chemistry, Multidisciplinary)The first ionization potential (IP ) yields information on valence electronic structure of an atom. IP
) yields information on valence electronic structure of an atom. IP values of heavy actinides beyond einsteinium (Es, Z = 99), however, have not been determined experimentally so far due to the difficulty in obtaining these elements on scales of more than one atom at a time. Recently, we successfully measured IP
values of heavy actinides beyond einsteinium (Es, Z = 99), however, have not been determined experimentally so far due to the difficulty in obtaining these elements on scales of more than one atom at a time. Recently, we successfully measured IP of lawrencium (Lr, Z = 103) using a surface ionization method. The result suggests that Lr has a loosely-bound electron in the outermost orbital. In contrast to Lr, nobelium (No, Z = 102) is expected to have the highest IP
of lawrencium (Lr, Z = 103) using a surface ionization method. The result suggests that Lr has a loosely-bound electron in the outermost orbital. In contrast to Lr, nobelium (No, Z = 102) is expected to have the highest IP among the actinide elements owing to its full-filled 5f and the 7s orbitals. In the present study, we have successfully determined IP
among the actinide elements owing to its full-filled 5f and the 7s orbitals. In the present study, we have successfully determined IP values of No as well as fermium (Fm, Z = 100) and mendelevium (Md, Z = 101) using the surface ionization method. The obtained results indicate that the IP
values of No as well as fermium (Fm, Z = 100) and mendelevium (Md, Z = 101) using the surface ionization method. The obtained results indicate that the IP value of heavy actinoids would increase monotonically with filling electrons up in the 5f orbital like heavy lanthanoids.
value of heavy actinoids would increase monotonically with filling electrons up in the 5f orbital like heavy lanthanoids.
Sato, Tetsuya; Asai, Masato; Borschevsky, A.*; Stora, T.*; Sato, Nozomi*; Kaneya, Yusuke; Tsukada, Kazuaki; D llmann, C. E.*; Eberhardt, K.*; Eliav, E.*; et al.
llmann, C. E.*; Eberhardt, K.*; Eliav, E.*; et al.
EPJ Web of Conferences, 131, p.05001_1 - 05001_6, 2016/12
Times Cited Count:0 Percentile:0.00(Chemistry, Inorganic & Nuclear)Ionization efficiency in a surface ionization process depends on the first ionization potential of the atom. Based on the dependence, the ionization potential of the atom can be determined. We measured ionization efficiencies of fermium, einsteinium, mendelevium, and lawrencium by using a newly developed gas-jet coupled surface ion-source. The ionization potential of the elements have not been determined so far due to their low production rates and/or their short half-lives. Based on a relationship between the ionization efficiency and the ionization potential obtained via measurements of short-lived lanthanide isotopes, the ionization potentials of these actinide elements have been successfully determined.
Sato, Tetsuya; Asai, Masato; Borschevsky, A.*; Stora, T.*; Sato, Nozomi; Kaneya, Yusuke; Tsukada, Kazuaki; D llmann, Ch. E.*; Eberhardt, K.*; Eliav, E.*; et al.
llmann, Ch. E.*; Eberhardt, K.*; Eliav, E.*; et al.
Nature, 520(7546), p.209 - 211, 2015/04
Times Cited Count:112 Percentile:97.06(Multidisciplinary Sciences)Ionization efficiency in a surface ionization process depends on the first ionization potential of the atom. Based on the dependence, the ionization potential of the atom can be determined. We successfully measured ionization efficiencies of lawrencium (Lr, 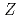 =103) using a gas-jet coupled surface ion-source. The ionization potential of Lr has not been determined owing to its low production rate and its short half-life. Based on a relationship between the ionization efficiency and the ionization potential obtained via measurements of short-lived lanthanide isotopes, the ionization potential of Lr was determined.
=103) using a gas-jet coupled surface ion-source. The ionization potential of Lr has not been determined owing to its low production rate and its short half-life. Based on a relationship between the ionization efficiency and the ionization potential obtained via measurements of short-lived lanthanide isotopes, the ionization potential of Lr was determined.
Rothe, S.*; Andreyev, A. N.*; Antalic, S.*; Borschevsky, A.*; Capponi, L.*; Cocolios, T. E.*; De Witte, H.*; Eliav, E.*; Fedorov, D. V.*; Fedosseev, V. N.*; et al.
Nature Communications (Internet), 4, p.1835_1 - 1835_6, 2013/05
Times Cited Count:93 Percentile:94.70(Multidisciplinary Sciences)Sato, Tetsuya; Asai, Masato; Kaneya, Yusuke*; Tsukada, Kazuaki; Toyoshima, Atsushi; Mitsukai, Akina*; Takeda, Shinsaku*; Vascon, A.*; Sakama, Minoru*; Sato, Daisuke*; et al.
no journal, ,
The first ionization potential (IP ) yields information on valence electronic structure of an atom. IP
) yields information on valence electronic structure of an atom. IP values of heavy actinides beyond einsteinium (Es, Z = 99), however, have not been determined experimentally so far due to the difficulty in obtaining these elements on scales of more than one atom at a time. Recently, we successfully measured IP
values of heavy actinides beyond einsteinium (Es, Z = 99), however, have not been determined experimentally so far due to the difficulty in obtaining these elements on scales of more than one atom at a time. Recently, we successfully measured IP of lawrencium (Lr, Z = 103) using a surface ionization method. The result suggests that Lr has a loosely-bound electron in the outermost orbital. In contrast to Lr, nobelium (No, Z = 102) is expected to have the highest IP
of lawrencium (Lr, Z = 103) using a surface ionization method. The result suggests that Lr has a loosely-bound electron in the outermost orbital. In contrast to Lr, nobelium (No, Z = 102) is expected to have the highest IP among the actinide elements owing to its full-filled 5f and the 7s orbitals. In the present study, we have successfully determined IP
among the actinide elements owing to its full-filled 5f and the 7s orbitals. In the present study, we have successfully determined IP values of No as well as fermium (Fm, Z = 100) and mendelevium (Md, Z = 101) using the surface ionization method. The obtained results indicate that the IP
values of No as well as fermium (Fm, Z = 100) and mendelevium (Md, Z = 101) using the surface ionization method. The obtained results indicate that the IP value of heavy actinoids would increase monotonically with filling electrons up in the 5f orbital like heavy lanthanoids.
value of heavy actinoids would increase monotonically with filling electrons up in the 5f orbital like heavy lanthanoids.
Nagame, Yuichiro; Sato, Tetsuya; Asai, Masato; Kaneya, Yusuke*; Makii, Hiroyuki; Mitsukai, Akina; Osa, Akihiko; Sch del, M.*; Toyoshima, Atsushi; Tsukada, Kazuaki; et al.
del, M.*; Toyoshima, Atsushi; Tsukada, Kazuaki; et al.
no journal, ,
Sato, Tetsuya; Asai, Masato; Kaneya, Yusuke*; Tsukada, Kazuaki; Toyoshima, Atsushi; Mitsukai, Akina*; Takeda, Shinsaku*; Vascon, A.*; Sakama, Minoru*; Sato, Daisuke*; et al.
no journal, ,
The first ionization potential (IP ) yields information on valence electronic structure of an atom. IP
) yields information on valence electronic structure of an atom. IP values of heavy actinides beyond einsteinium (Es, Z = 99), however, have not been determined experimentally so far due to the difficulty in obtaining these elements on scales of more than one atom at a time. Recently, we successfully measured IP
values of heavy actinides beyond einsteinium (Es, Z = 99), however, have not been determined experimentally so far due to the difficulty in obtaining these elements on scales of more than one atom at a time. Recently, we successfully measured IP of lawrencium (Lr, Z = 103) using a surface ionization method. The result suggests that Lr has a loosely-bound electron in the outermost orbital. In contrast to Lr, nobelium (No, Z = 102) is expected to have the highest IP
of lawrencium (Lr, Z = 103) using a surface ionization method. The result suggests that Lr has a loosely-bound electron in the outermost orbital. In contrast to Lr, nobelium (No, Z = 102) is expected to have the highest IP among the actinide elements owing to its full-filled 5f and the 7s orbitals. In the present study, we have successfully determined IP
among the actinide elements owing to its full-filled 5f and the 7s orbitals. In the present study, we have successfully determined IP values of No as well as fermium (Fm, Z = 100) and mendelevium (Md, Z = 101) using the surface ionization method. The obtained results indicate that the IP
values of No as well as fermium (Fm, Z = 100) and mendelevium (Md, Z = 101) using the surface ionization method. The obtained results indicate that the IP value of heavy actinoids would increase monotonically with filling electrons up in the 5f orbital like heavy lanthanoids.
value of heavy actinoids would increase monotonically with filling electrons up in the 5f orbital like heavy lanthanoids.
Sato, Tetsuya; Stora, T.*; Asai, Masato; Borschevsky, A.*; Kaneya, Yusuke; Tsukada, Kazuaki; D llmann, Ch. E.*; Eliav, E.*; Kaldor, U.*; Kratz, J. V.*; et al.
llmann, Ch. E.*; Eliav, E.*; Kaldor, U.*; Kratz, J. V.*; et al.
no journal, ,
It is known that an ionization efficiency of an element in surface ionization process depends on temperature, work function of the surface, and the first ionization energy of the atom of the element to be ionized. We determined the first ionization energy of lawrencium (Lr), the last member of the actinide series, using the relationship between the ionization efficiency and the ionization energy. The ionization energy of Lr had not been measured owing to its low production rate and short half-lives so far. The experimental value of the ionization energy is in good agreement of the theoretical one which was calculated using the state-of-the-art relativistic calculation.