Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Maamoun, I.; Falyouna, O.*; Shariful, I. M.*; Eljamal, R.*; Bensaida, K.*; 田中 万也; 徳永 紘平; Eljamal, O.*
Advanced Materials Letters (Internet), 14(2), p.23021721_1 - 23021721_10, 2023/04
Arsenic (As) contamination of water has been considered as an issue of great concern. In this study, we developed magnesium hydroxide coated iron nanoparticles (nFe0@Mg(OH) ) for enhancing arsenic removal from aqueous solutions. For a practical use, several parameters were investigated, including Mg/Fe coating ratio, nFe0@Mg(OH)
) for enhancing arsenic removal from aqueous solutions. For a practical use, several parameters were investigated, including Mg/Fe coating ratio, nFe0@Mg(OH) dosage, initial pH, reaction temperature, and initial As(V) concentration.
dosage, initial pH, reaction temperature, and initial As(V) concentration.
 bimetallic Ni/Fe
bimetallic Ni/Fe nanoparticles; Implications towards Tc(VII) removal
nanoparticles; Implications towards Tc(VII) removalMaamoun, I.; 徳永 紘平; 土肥 輝美; 菅野 太志*; Falyouna, O.*; Eljamal, O.*; 田中 万也
Frontiers in Nuclear Engineering (Internet), 2, p.1142823_1 - 1142823_17, 2023/03
テクネチウム99は半減期が長く易動性の高い放射性核種であることから、固相として回収することが困難であるとされてきた。本研究では、新たにFe -Niナノ粒子のTc(VII)除去へ有効性を評価するために、アナログ元素であるRe(VII)の除去実験を行った。その結果、Fe
-Niナノ粒子のTc(VII)除去へ有効性を評価するために、アナログ元素であるRe(VII)の除去実験を行った。その結果、Fe -Niナノ粒子を用いて高い効率で水溶液中からRe(VII)を回収できることが示された。
-Niナノ粒子を用いて高い効率で水溶液中からRe(VII)を回収できることが示された。
Maamoun, I.; Rushdi, M.*; Falyouna, O.*; Eljamal, R.*; Eljamal, O.*
Separation and Purification Technology, 308, p.122863_1 - 122863_16, 2023/03
被引用回数:9 パーセンタイル:42.05(Engineering, Chemical)The aim of this study is to employ machine learning (ML) in providing high-accuracy prediction of Cr(VI) removal efficiency by nickel hydroxide ( -Ni(OH)
-Ni(OH) ) unconventional sorbent, towards the new era of artificial intelligence (AI) applications in (waste) water treatment. Hence, a reliable ML modeling was conducted based on the experimental investigation, considering different reaction parameters, including
) unconventional sorbent, towards the new era of artificial intelligence (AI) applications in (waste) water treatment. Hence, a reliable ML modeling was conducted based on the experimental investigation, considering different reaction parameters, including  -Ni(OH)
-Ni(OH) dosage, initial pH, reaction temperature, and initial Cr(VI) concentration. Linear regression model was selected as the suitable regression model with respect to the obtained reasonable correlation and the less training time and evaluation time, comparing to other considered regression techniques. The adopted linear regression model, for the time corresponding Cr(VI) removal efficiencies, exhibited satisfactory prediction accuracy. Furthermore, the importance of models coefficients was determined and implied the high importance of the dosage feature. The contributive effect of the investigated features was mainly concentrated at the early stage of the reaction (5 to 10 min), with an average range of 50 to 80 %, which was in agreement with the experimental findings of the rapid and full removal of Cr(VI) by
dosage, initial pH, reaction temperature, and initial Cr(VI) concentration. Linear regression model was selected as the suitable regression model with respect to the obtained reasonable correlation and the less training time and evaluation time, comparing to other considered regression techniques. The adopted linear regression model, for the time corresponding Cr(VI) removal efficiencies, exhibited satisfactory prediction accuracy. Furthermore, the importance of models coefficients was determined and implied the high importance of the dosage feature. The contributive effect of the investigated features was mainly concentrated at the early stage of the reaction (5 to 10 min), with an average range of 50 to 80 %, which was in agreement with the experimental findings of the rapid and full removal of Cr(VI) by  -Ni(OH)
-Ni(OH) . The elucidated insights into the effects of different factors that influence Cr(VI) removal process by
. The elucidated insights into the effects of different factors that influence Cr(VI) removal process by  -Ni(OH)
-Ni(OH) revealed the underlying interactions and removal pathways, which shall benefit other researchers in the preliminary design of pilot-scale applications and anticipating the predicted performance.
revealed the underlying interactions and removal pathways, which shall benefit other researchers in the preliminary design of pilot-scale applications and anticipating the predicted performance.
 @Mg(OH)
@Mg(OH) ) into porous media for Cr(VI) removal from groundwater
) into porous media for Cr(VI) removal from groundwaterMaamoun, I.; Falyouna, O.*; Eljamal, R.*; Idham, M. F.*; 田中 万也; Eljamal, O.*
Chemical Engineering Journal, 451, Part3, p.138718_1 - 138718_22, 2023/01
被引用回数:44 パーセンタイル:92.88(Engineering, Environmental)Chromium (VI) contamination in groundwater represents a significant threat to the current and future groundwater resources. Thus, in this work detailed investigation was conducted on the injection of magnesium hydroxide encapsulated iron nanoparticles (nFe @Mg(OH)
@Mg(OH) ) into a 3-D bench-scale groundwater treatment system for Cr(VI) removal. Cr(VI) and total iron concentration profiles were determined for the injection of both nFe
) into a 3-D bench-scale groundwater treatment system for Cr(VI) removal. Cr(VI) and total iron concentration profiles were determined for the injection of both nFe and nFe
and nFe @Mg(OH)
@Mg(OH) into porous media. The results indicated the expected poor mobility of nFe
into porous media. The results indicated the expected poor mobility of nFe , which caused the accumulation of the injected mass within the injection zone and the low spreading range along the length of the aquifer. The injection of nFe
, which caused the accumulation of the injected mass within the injection zone and the low spreading range along the length of the aquifer. The injection of nFe @Mg(OH)
@Mg(OH) into the groundwater treatment system for 80 consecutive cycles resulted in a clear enhancement in preventing the rapid corrosion of the iron core and around twenty percent improvement in the final Cr(VI) removal efficiency compared with that of nFe
into the groundwater treatment system for 80 consecutive cycles resulted in a clear enhancement in preventing the rapid corrosion of the iron core and around twenty percent improvement in the final Cr(VI) removal efficiency compared with that of nFe . The injected nFe
. The injected nFe @Mg(OH)
@Mg(OH) maintained the full Cr(VI) removal efficiency for 30 post-injection cycles. Such a promising potential of the nFe
maintained the full Cr(VI) removal efficiency for 30 post-injection cycles. Such a promising potential of the nFe @Mg(OH)
@Mg(OH) , proposed it as one of the perfect candidates for in-situ water treatment applications, as a reactive nanomaterial with enhanced features, in terms of the prolonged reactive performance and the widespread of the injection zone to cover a larger contaminated area within the porous media.
, proposed it as one of the perfect candidates for in-situ water treatment applications, as a reactive nanomaterial with enhanced features, in terms of the prolonged reactive performance and the widespread of the injection zone to cover a larger contaminated area within the porous media.
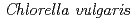 and nano zero-valent iron
and nano zero-valent ironIslam, M. S.*; Maamoun, I.; Falyouna, O.*; Eljamal, O.*; Saha, B. B.*
Journal of Molecular Liquids, 370, p.121005_1 - 121005_11, 2023/01
被引用回数:41 パーセンタイル:98.41(Chemistry, Physical)Arsenic waste must be carefully managed because of the adverse effects of arsenic in wastewater on the ecosystem. In the present study, an environmentally friendly novel composite of 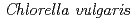 microalgae and nano-zero valent iron (NZVI) was employed as an adsorbent to eliminate arsenic from the aqueous environment. Fourier Transform Infrared spectroscopy, X-ray diffraction, and scanning electron microscope images were used to characterize and analyze the CV/NZVI composites. Batch tests using initial arsenic concentrations ranging from 5 to 100 mg/L were conducted to evaluate removal efficiencies. According to kinetic analysis, the best model for fitting the experimental data was the pseudo first-order model, which had the lowest Akaike information criterion (AIC), and Bayesian information criterion (BIC) values of -23.878 and -7.902, respectively. Results alluded that physisorption is the primary mechanism influenced by As-removal by CV/NZVI composite. Due to the negative sign of the enthalpy and Gibbs free energy, the thermodynamic investigation revealed that the adsorption reaction was exothermic and spontaneous. The thermodynamic analysis also affirmed that the arsenic removal process involved primarily physisorption and slight chemisorption phenomena. Meanwhile, 1.5 g/L CV/NZVI dosage achieved 99% As(V) removal efficiency in synthetic groundwater systems, confirming the high potential of the composite in complex aqueous systems.
microalgae and nano-zero valent iron (NZVI) was employed as an adsorbent to eliminate arsenic from the aqueous environment. Fourier Transform Infrared spectroscopy, X-ray diffraction, and scanning electron microscope images were used to characterize and analyze the CV/NZVI composites. Batch tests using initial arsenic concentrations ranging from 5 to 100 mg/L were conducted to evaluate removal efficiencies. According to kinetic analysis, the best model for fitting the experimental data was the pseudo first-order model, which had the lowest Akaike information criterion (AIC), and Bayesian information criterion (BIC) values of -23.878 and -7.902, respectively. Results alluded that physisorption is the primary mechanism influenced by As-removal by CV/NZVI composite. Due to the negative sign of the enthalpy and Gibbs free energy, the thermodynamic investigation revealed that the adsorption reaction was exothermic and spontaneous. The thermodynamic analysis also affirmed that the arsenic removal process involved primarily physisorption and slight chemisorption phenomena. Meanwhile, 1.5 g/L CV/NZVI dosage achieved 99% As(V) removal efficiency in synthetic groundwater systems, confirming the high potential of the composite in complex aqueous systems.
Falyouna, O.*; Maamoun, I.; Ghosh, S.*; Malloum, A.*; Othmani, A.*; Eljamal, O.*; Amen, T. W. M.*; Oroke, A.*; Bornman, C.*; Ahmadi, S.*; et al.
Journal of Molecular Liquids, 368, Part B, p.120726_1 - 120726_25, 2022/12
被引用回数:14 パーセンタイル:49.79(Chemistry, Physical)Despite the carcinogenic and other adverse health effects ofchloramphenicol (CAP), it is frequently detected in different water sources (e.g., groundwater, surface water, wastewater effluents, etc.) due to ongoing, illegal, and abusive application of CAP in veterinary medicine. Although extensive research has been carried out to develop effective treatment technologies to remove the persistent CAP from aqueous mediums, yet there is no critical review of these studies to the best of our reach This review will be the first in the literature to comprehensively summarize the state-of-the-art treatment techniques for CAP removal from water. We report the removal of CAP by adsorption, biodegradation, nanoscale zerovalent iron technology (nZVI), and advanced oxidation processes (AOPs). The result shows that carbon-based adsorbents have more q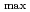 equal 892.86 mg/g for Porous carbon material from
equal 892.86 mg/g for Porous carbon material from 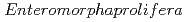 . The Langmuir- Freundlich isotherm and pseudo-second order kinetics model were reported to best describe the isotherm and kinetic model respectively. Removing the CAP via biodegradation would achieve the advantages of low operating costs, and environmental friendliness. The process of AOPs among the various treatment options can be a promising method for CAP degradation in water. This review comprehensively summarizes the state-of-the-art treatment techniques for CAP removal from water. Particularly, serving as an inclusive reference for future researchers to easily define the research gabs in the literature and plan for their future work in developing novel treatment methods to decontaminate CA-contaminated waters.
. The Langmuir- Freundlich isotherm and pseudo-second order kinetics model were reported to best describe the isotherm and kinetic model respectively. Removing the CAP via biodegradation would achieve the advantages of low operating costs, and environmental friendliness. The process of AOPs among the various treatment options can be a promising method for CAP degradation in water. This review comprehensively summarizes the state-of-the-art treatment techniques for CAP removal from water. Particularly, serving as an inclusive reference for future researchers to easily define the research gabs in the literature and plan for their future work in developing novel treatment methods to decontaminate CA-contaminated waters.
Idham, M. F.*; Falyouna, O.*; Eljamal, R.*; Maamoun, I.; Eljamal, O.*
Journal of Water Process Engineering (Internet), 50, p.103289_1 - 103289_16, 2022/12
被引用回数:35 パーセンタイル:95.02(Engineering, Environmental)Due to synthesis variation affecting various graphene oxide (GO) physicochemical parameters and cost efficiency aspects, the present study investigated the influence of GO precursor components for GO precipitated nZVI nanocomposite (nZVI/GO) and optimized removal conditions to remove chloramphenicol (CAP) from water. In order to synthesize nZVI/GO nanocomposites, four methods of GO precursor synthesis were used, denoted GO1, GO2, GO3, and GO4. A novel synthesis process is introduced based on economic and time-less-consuming protocols to produce GO precursor. A series of desorption experiments were also implemented in various eluents to clarify the CAP removal mechanism. Interestingly, this study demonstrated the substantial impact of GO precursor on the nanocomposite performance in eliminating CAP. The introduced novel GO successfully served as an excellent nZVI precipitation medium and enhanced CAP removal efficiency. Empirical optimization demonstrated that nZVI/GO4-1:1 could eliminate up to 91% of 100 mg/L CAP by dosage as low as 0.25 g/L at pH 5. nZVI/GO4 displayed CAP removal stability throughout a more comprehensive pH range, and remarkable recyclability, making it more promising and practical than bare nZVI and other analyzed nanocomposites. Kinetics data demonstrated a high degree of compatibility with the pseudo-first-order (PFO) and pseudo-second-order (PSO). Through kinetics and statistical analyses, desorption experiments, FTIR spectroscopy, and EDX analysis, nZVI/GO4 removed some of the CAP through the adsorption mechanism controlled by physisorption and chemisorption. In contrast, the oxidation mechanism eliminated the remaining CAP.
Falyouna, O.*; Idham, M. F.*; Maamoun, I.; Bensaida, K.*; Ashik, U. P. M.*; 杉原 裕司*; Eljamal, O.*
Journal of Molecular Liquids, 359, p.119323_1 - 119323_20, 2022/08
被引用回数:49 パーセンタイル:98.94(Chemistry, Physical)Water contamination by ciprofloxacin (CIP) is a global and emerging issue because it increases the risk of infection by antimicrobial resistant bacteria. CIP removal from water by iron nanoparticles (Fe ) with the presence of oxalate hasn't been reported yet. The present study demonstrated that the addition of oxalate to Fe
) with the presence of oxalate hasn't been reported yet. The present study demonstrated that the addition of oxalate to Fe nanoparticles improved the removal of CIP under the following optimum conditions: dose = 0.3 g L
nanoparticles improved the removal of CIP under the following optimum conditions: dose = 0.3 g L , oxalate = 0.3 mM, initial pH = 7, and temperature = 25
, oxalate = 0.3 mM, initial pH = 7, and temperature = 25  C. Furthermore, the experimental results illustrated that high concentrations of dissolved oxygen in the aqueous solution greatly decreased the removal efficiency of CIP by Fe
C. Furthermore, the experimental results illustrated that high concentrations of dissolved oxygen in the aqueous solution greatly decreased the removal efficiency of CIP by Fe oxalate system. In addition, the desorption experiments and the results of SEM-EDS, XRD, and FTIR revealed that physisorption and chemisorption were responsible for CIP removal by Fe
oxalate system. In addition, the desorption experiments and the results of SEM-EDS, XRD, and FTIR revealed that physisorption and chemisorption were responsible for CIP removal by Fe oxalate system as the addition of 0.3 mM of oxalate boosted the surface complexation between Fe
oxalate system as the addition of 0.3 mM of oxalate boosted the surface complexation between Fe nanoparticles and the carboxylic, ketone, and piperazinyl groups in CIP. These results were supported by the outcomes of kinetics, isotherm, and thermodynamic analysis. Finally, this study proved that Fe
nanoparticles and the carboxylic, ketone, and piperazinyl groups in CIP. These results were supported by the outcomes of kinetics, isotherm, and thermodynamic analysis. Finally, this study proved that Fe oxalate system is inexpensive, practical, and more efficient than most of the reported Fe
oxalate system is inexpensive, practical, and more efficient than most of the reported Fe -based systems with a maximum adsorption capacity of 294.66 mg g
-based systems with a maximum adsorption capacity of 294.66 mg g .
.
Maamoun, I.; Bensaida, K.*; Eljamal, R.*; Falyouna, O.*; 田中 万也; Tosco, T.*; 杉原 裕司*; Eljamal, O.*
Journal of Molecular Liquids, 358, p.119216_1 - 119216_13, 2022/07
被引用回数:40 パーセンタイル:98.30(Chemistry, Physical)In this study, nickel hydroxide nanoplates (nNiHs) were developed to achieve rapid and significant Cr(VI) removal from aqueous solutions. nNiHs showed an average particle size and crystallite size of 36.8 nm and 8.68 nm, respectively. Different reaction parameters were investigated, including nNiHs dosage, pH, reaction temperature, initial Cr(VI) concentration, and co-existing anions. nNiHs could efficiently remove 20 mg/L Cr(VI) concentration over a wide pH and temperature range(s). Pseudo 2nd order kinetic model and Freundlich isotherm model were the best to fit experimental data. A maximum Cr(VI) sorption capacity of 71.25 mg/g was achieved at the optimal reaction conditions, comparable to the previously reported values. The governing Cr(VI) removal mechanism by nNiHs involved the high dominance of electrostatic adsorption and the low dominance of co-precipitation. The high sorption potential of the nNiHs and the high affinity of the aqueous Cr(VI) species, enabled the proposed adsorbent to yield an efficient performance in binary environmental systems.
Maamoun, I.; Falyouna, O.*; Eljamal, R.*; Bensaida, K.*; 田中 万也; Tosco, T.*; 杉原 裕司*; Eljamal, O.*
Journal of Environmental Chemical Engineering, 10(3), p.107431_1 - 107431_17, 2022/06
被引用回数:46 パーセンタイル:93.56(Engineering, Environmental)In this study, the reactive performance of magnesium hydroxide-coated iron nanoparticles was investigated for the removal of hexavalent chromium from aqueous solutions. Short- and long-term progressive-release of reactivity was evaluated through several batch tests. The Multi-functional effect of the environmentally-friendly magnesium hydroxide coating shell was represented by the progressive shell-dissolution in water and preventing the rapid corrosion of the iron core, which resulted in a controlled release of reactivity towards hexavalent chromium. Magnesium hydroxide-coated iron nanoparticles showed good performance in preserving the long-term reactivity within a wide ranges of pH and temperature. The long-term investigation of magnesium hydroxide-coated iron nanoparticles performance towards hexavalent chromium removal confirmed the progressive and maintained reactivity, represented by the continuous release of iron core electrons, to achieve full removal over 50 days reaction time, to be reported for the first time in the literature. The material showed high regeneration abilities up to 5 cycles with 1.36 times average enhancement in hexavalent chromium removal efficiency compared to that of iron. Moreover, it achieved an increase in the shelf-live longevity performance up to 30 days without any storing solution with considerable removal efficiency after 180 min reaction time.
Eljamal, O.*; Maamoun, I.; Alkhudhayri, S.*; Eljamal, R.*; Falyouna, O.*; 田中 万也; 香西 直文; 杉原 裕司*
Journal of Water Process Engineering (Internet), 46, p.102608_1 - 102608_13, 2022/04
被引用回数:46 パーセンタイル:97.52(Engineering, Environmental)In this study, calcined Mg-Al layered double hydroxide was successfully synthesized for boron removal from aqueous solutions. Batch experiments were conducted considering various reaction conditions, including initial pH, reaction temperature, initial boron concentration, Mg-Al-CLDH dosage, ambient condition, and co-existing ions effect, for optimizing boron removal efficiency. Results showed that sorption kinetic rate became higher by approaching towards the neutral pH conditions, while it declined at the strong acidic or alkaline conditions. Mg-Al-CLDH was capable of removing high boron concentration from aqueous solutions at a reasonable dosage, with a comparable sorption capacity to the other reported studies. Moreover, high boron removal rates were observed at high reaction temperatures, reflecting the endothermic nature of the reaction, and reached equilibrium within less than 6 h. Moreover, results of 3D-RSM modeling confirmed that the middle-high range of Mg-Al-CLDH dosage values was the suitable range to achieve high boron removal efficiency, in spite of pH, temperature, and initial concentration effects. Furthermore, isotherm modeling confirmed that boron removal by Mg-Al-CLDH occurred via a mono-layer sorption, and thermodynamic modeling revealed the positive value of entropy change, indicating that the randomness of the solid/liquid interaction increased within the adsorption process of boron. Spent Mg-Al-CLDH showed great reusability performance by considerable boron removal efficiency over three consecutive regeneration cycles, confirming the high potential and applicability of the presented adsorbent in real water treatment applications.
 /MgO] for prompt and efficient adsorption of ciprofloxacin from aqueous solutions
/MgO] for prompt and efficient adsorption of ciprofloxacin from aqueous solutionsFalyouna, O.*; Bensaida, K.*; Maamoun, I.; Ashik, U. P. M.*; 田原 淳士*; 田中 万也; 青柳 登; 杉原 裕司*; Eljamal, O.*
Journal of Cleaner Production, 342, p.130949_1 - 130949_15, 2022/03
被引用回数:62 パーセンタイル:98.17(Green & Sustainable Science & Technology)The antibiotic ciprofloxacin is recognized as a contaminant of emerging concern because its persistent occurrence in water accelerates the growth of deadly antimicrobial resistance genes. For the first time, the conventional precipitation technique was thermally modified to produce hybrid magnesium hydroxide/magnesium oxide nanorods for efficient and rapid adsorption of CIP from water. The successful synthesis was confirmed by the outcomes of TEM, EDS, XRD, and FTIR analysis. Mg(OH) /MgO exhibited an extraordinary capability to adsorb CIP from water regardless of CIP initial concentration under neutral pH and room temperature. FTIR analysis for the spent Mg(OH)
/MgO exhibited an extraordinary capability to adsorb CIP from water regardless of CIP initial concentration under neutral pH and room temperature. FTIR analysis for the spent Mg(OH) /MgO revealed that bridging complexation with carboxylic group and electrostatic attraction with the positive amine group are the responsible mechanisms for CIP adsorption by Mg(OH)
/MgO revealed that bridging complexation with carboxylic group and electrostatic attraction with the positive amine group are the responsible mechanisms for CIP adsorption by Mg(OH) /MgO. Moreover, simulated CIP-contaminated river water was efficiently treated by Mg (OH)
/MgO. Moreover, simulated CIP-contaminated river water was efficiently treated by Mg (OH) /MgO which proves the promising performance of Mg(OH)
/MgO which proves the promising performance of Mg(OH) /MgO in field scale applications.
/MgO in field scale applications.
Bensaida, K.*; Maamoun, I.; Eljamal, R.*; Falyouna, O.*; 杉原 裕司*; Eljamal, O.*
Energy Conversion and Management, 249, p.114877_1 - 114877_12, 2021/12
被引用回数:44 パーセンタイル:93.63(Thermodynamics)Microbial fuel cells (MFC) are a versatile technology for power generation from biodegradable solid wastes. This study examines the addition of bare and coated Fe0 nanoparticles to the anolyte of a lab-scale MFC for the first time. Four different coating ratios (0.1, 0.2, 0.5, and 1.0) were separately added and comparatively evaluated for power generation. The study examined the use of four different waste sludge substrates, different pH, and aerobic enriched cathode chambers effect on wastewater treatment and current production. Results showed that coating ratio of 0.2 was promising to achieve 4 times increase in the voltage compared to the control and provide the maximal power density. The current generation stability was achieved under neutral pH, and the power density output is maintained high under anaerobic conditions. The addition of the coated Fe0 nanoparticles is an effective method to enhance electricity generation and sludge digestion. However, additional parameters should be considered.
Maamoun, I.; Falyouna, O.*; Shariful, I. M.*; Eljamal, R.*; Bensaida, K.*; 田中 万也; 徳永 紘平; Eljamal, O.*
no journal, ,
The main aim of this study is to investigate the potential of Mg(OH) coated iron nanoparticles in achieving improved arsenic removal from aqueous solutions. Set of batch tests has been conducted to understand the effect of several reaction factors, including coating ratio optimization, dosage, initial pH, temperature, and initial As(V) concentration. Results indicated that full coating was the optimal Mg(OH)
coated iron nanoparticles in achieving improved arsenic removal from aqueous solutions. Set of batch tests has been conducted to understand the effect of several reaction factors, including coating ratio optimization, dosage, initial pH, temperature, and initial As(V) concentration. Results indicated that full coating was the optimal Mg(OH) coating ratio which yielded full removal efficiency after 120 min reaction time, higher than that of nFe0 and Mg(OH)
coating ratio which yielded full removal efficiency after 120 min reaction time, higher than that of nFe0 and Mg(OH) coated iron nanoparticles with lower coating ratios. Furthermore, both strong acidic and high temperature conditions were favorable for inducing the arsenic removal performance of Mg(OH)
coated iron nanoparticles with lower coating ratios. Furthermore, both strong acidic and high temperature conditions were favorable for inducing the arsenic removal performance of Mg(OH) coated iron nanoparticles. Still, Mg(OH)
coated iron nanoparticles. Still, Mg(OH) coated iron nanoparticles could efficiently achieve comparable removal at a wide pH and temperature ranges. Such results implied the contribution of Mg(OH)
coated iron nanoparticles could efficiently achieve comparable removal at a wide pH and temperature ranges. Such results implied the contribution of Mg(OH) to As(V) removal via adsorption and the possible co-precipitation of As(III) with the released Mg
to As(V) removal via adsorption and the possible co-precipitation of As(III) with the released Mg from the coating shell dissolution. Besides, the progressive release of electrons from the iron core contributed to As(V) reduction to As(III). In conclusion, the proposed Mg(OH)
from the coating shell dissolution. Besides, the progressive release of electrons from the iron core contributed to As(V) reduction to As(III). In conclusion, the proposed Mg(OH) coated iron nanoparticles could be a perfect nanomaterial candidate for the real applications of arsenic removal from contaminated waters.
coated iron nanoparticles could be a perfect nanomaterial candidate for the real applications of arsenic removal from contaminated waters.

 ) removal from aqueous solutions by mono-, bi-, and tri-metallic iron nanoparticles; A Comparative study
) removal from aqueous solutions by mono-, bi-, and tri-metallic iron nanoparticles; A Comparative studyMaamoun, I.; 徳永 紘平; Falyouna, O.*; Eljamal, O.*; 田中 万也
no journal, ,
Recently, the rapid development of nuclear power technologies and the continuous energy demand around the world exhibited massive amounts of contaminated water with radionuclides. The exposure to TcVII-contaminated water can be harmful to human health, causing toxic effects and organs damage when ingested. Therefore, TcO 
 removal from aqueous solutions can be challenging, in terms of fast and efficient immobilization. Correspondingly, perrhenate (ReO
removal from aqueous solutions can be challenging, in terms of fast and efficient immobilization. Correspondingly, perrhenate (ReO
 ) was considered as perrhenate (TcO
) was considered as perrhenate (TcO
 ) surrogate to ease the radioactivity-related complications, owing to the physiochemical similarities between Tc and rhenium (Re). In this study, nickel (Ni) andzirconium (Zr) were considered in the preparation of bi- and tri-metallic Fe0 nanoparticles, as they both showed the highest ReO
) surrogate to ease the radioactivity-related complications, owing to the physiochemical similarities between Tc and rhenium (Re). In this study, nickel (Ni) andzirconium (Zr) were considered in the preparation of bi- and tri-metallic Fe0 nanoparticles, as they both showed the highest ReO 
 removal performance comparing with other metals. The effect of reaction conditions on ReO
removal performance comparing with other metals. The effect of reaction conditions on ReO 
 removal was investigated, including mass ratio of iron to the doped metal, material dosage, and initial pH. Results showed enhanced ReO
removal was investigated, including mass ratio of iron to the doped metal, material dosage, and initial pH. Results showed enhanced ReO 
 removal rate when using bi-metallic Ni-Fe0 (mass ratio 2.5) and Zr/Fe0 (mass ratio 20) comparing with Fe0. The difference in ReO
removal rate when using bi-metallic Ni-Fe0 (mass ratio 2.5) and Zr/Fe0 (mass ratio 20) comparing with Fe0. The difference in ReO
 removal using mono-, bi-, and tri-metallic was not clear at high material dosage, such as 2.0 and 1.0 g/L. Nevertheless, comparing lower dosage (0.5 g/L)of bi- and tri-metallic to 1.0 g/L mono-metallic Fe0 dosage exhibited a clear superiority of tri-metallic Zr-Ni/Fe0 to other materials; where 0.5 g/L of the material could efficiently achieve around 98% ReO
removal using mono-, bi-, and tri-metallic was not clear at high material dosage, such as 2.0 and 1.0 g/L. Nevertheless, comparing lower dosage (0.5 g/L)of bi- and tri-metallic to 1.0 g/L mono-metallic Fe0 dosage exhibited a clear superiority of tri-metallic Zr-Ni/Fe0 to other materials; where 0.5 g/L of the material could efficiently achieve around 98% ReO
 removal within just 10 min reaction time (1.8 times higher than 1.0 g/L Fe0). The significant enhancement in ReO
removal within just 10 min reaction time (1.8 times higher than 1.0 g/L Fe0). The significant enhancement in ReO
 removal rate by tri-metallic Fe0 nanoparticles can be attributed to the induced rate of electron transfer from iron core through the mixed Zr-Nideposits on Fe0 surface.
removal rate by tri-metallic Fe0 nanoparticles can be attributed to the induced rate of electron transfer from iron core through the mixed Zr-Nideposits on Fe0 surface.