Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 , SO
, SO ) precipitates
) precipitatesRai, D.*; Felmy, A. R.*; Moore, D. A.*; Kitamura, Akira; Yoshikawa, Hideki; Doi, Reisuke; Yoshida, Yasushi*
Radiochimica Acta, 102(8), p.711 - 721, 2014/08
Times Cited Count:2 Percentile:16.44(Chemistry, Inorganic & Nuclear)The solubility of Ba(SeO , SO
, SO ) precipitates was determined as a function of the BaSeO
) precipitates was determined as a function of the BaSeO mole fractions, ranging from 0.0015 to 0.3830, and time with an equilibration period extending to as long as 302 days. Equilibrium/steady state conditions in this system are reached in
mole fractions, ranging from 0.0015 to 0.3830, and time with an equilibration period extending to as long as 302 days. Equilibrium/steady state conditions in this system are reached in 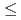 65 days. Pitzer's ion interaction model was used to calculate solid and aqueous phase activity coefficients. Thermodynamic analyses showed that the data do not satisfy Gibbs-Duhem equation, thereby demonstrating that a single-solid solution phase does not control both the selenate and sulfate concentrations. Our extensive data with [Ba], [SeO
65 days. Pitzer's ion interaction model was used to calculate solid and aqueous phase activity coefficients. Thermodynamic analyses showed that the data do not satisfy Gibbs-Duhem equation, thereby demonstrating that a single-solid solution phase does not control both the selenate and sulfate concentrations. Our extensive data with [Ba], [SeO ], and [SO
], and [SO ] can be explained with the formation of an ideal BaSeO
] can be explained with the formation of an ideal BaSeO solid solution phase that controls the selenium concentrations and a slightly disordered/less-crystalline BaSO
solid solution phase that controls the selenium concentrations and a slightly disordered/less-crystalline BaSO (s) that controls the sulfate concentrations. In these experiments the BaSO
(s) that controls the sulfate concentrations. In these experiments the BaSO component of the solid solution phase never reaches thermodynamic equilibrium with the aqueous phase. Thermodynamic interpretations of the data show that both the ideal BaSeO
component of the solid solution phase never reaches thermodynamic equilibrium with the aqueous phase. Thermodynamic interpretations of the data show that both the ideal BaSeO solid solution phase and less-crystalline BaSO
solid solution phase and less-crystalline BaSO (s) phase are in equilibrium with each other in the entire range of BaSeO
(s) phase are in equilibrium with each other in the entire range of BaSeO mole fractions investigated in this study.
mole fractions investigated in this study.
 (cr) in the aqueous Ba
(cr) in the aqueous Ba -SeO
-SeO
 -Na
-Na -H
-H -OH
-OH -H
-H O system; Extending to high selenate concentrations
O system; Extending to high selenate concentrationsRai, D.*; Felmy, A. R.*; Moore, D. A.*; Kitamura, Akira; Yoshikawa, Hideki; Doi, Reisuke; Yoshida, Yasushi*
Radiochimica Acta, 102(9), p.817 - 830, 2014/04
Times Cited Count:1 Percentile:8.88(Chemistry, Inorganic & Nuclear)The aqueous solubility of BaSeO (cr) was studied in Na
(cr) was studied in Na SeO
SeO solutions ranging in concentration from 0.0001 to 4.1 mol.kg
solutions ranging in concentration from 0.0001 to 4.1 mol.kg and maintained in a N
and maintained in a N atmosphere at room temperature (296
atmosphere at room temperature (296  2 K). The studies were conducted from both the undersaturation and oversaturation directions, with equilibration periods ranging from 3 to 596 days. The equilibrium in this system was reached rather rapidly (
2 K). The studies were conducted from both the undersaturation and oversaturation directions, with equilibration periods ranging from 3 to 596 days. The equilibrium in this system was reached rather rapidly (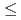 3 days). The SIT and Pitzer's ion-interaction models were used to interpret these data and the predictions based on both of these models agreed closely with the experimental data.
3 days). The SIT and Pitzer's ion-interaction models were used to interpret these data and the predictions based on both of these models agreed closely with the experimental data.
Rai, D.*; Rao, L.*; Weger, H. T.*; Felmy, A. R.*; Choppin, G. R.*; Yui, Mikazu
JNC TN8400 99-009, 115 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of Th(IV), U(IV), Np(IV), and Pu(IV) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. Where data for specific actinide(IV) species was lacking, the data were selected based on chemical analogy to other tetravalent actinides. ln this study, the Pitzer ion-interaction model is used to extrapolate thermodynamic constants to zero ionic strength at 25 C.
C.