Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yatsuka, Eiichi; Hatae, Takaki; Fujie, Daijiro*; Kurokawa, Atsuo*; Kusama, Yoshinori
Review of Scientific Instruments, 83(10), p.10E328_1 - 10E328_3, 2012/07
Times Cited Count:2 Percentile:11.68(Instruments & Instrumentation)On the edge Thomson scattering (TS) system in ITER, the filter polychromator is used for dispersing the scattered radiation. Since the signal in TS system is very small, it is crucial to reduce the signal loss from the collection optics to the spectrometer. A band of wavelength in the signal is extracted by an interference filter, and the remaining part is reflected at the interference filter. On the conventional polychromator, a number of reflections are necessary for extracting all wavelength bands. We proposed a new type of polychromator. On the new polychromator, both transmitted and reflected signal from an interference filter are dispersed into transmitted and reflected parts again. It enables to reduce the number of reflection at the interference filters. We showed it is possible to apply in the edge TS system in ITER by setting the injection angle of signal light into the interference filters to be approximately less than 10 degrees.
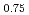 Sr
Sr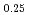 )MnO
)MnO and (Ba
and (Ba Sr
Sr )(Co
)(Co Fe
Fe )O
)O through rietveld refinement and MEM analysis
through rietveld refinement and MEM analysisIto, Takanori*; Shirasaki, Saori*; Fujie, Yoshinori*; Kitamura, Naoto*; Idemoto, Yasushi*; Osaka, Keiichi*; Hirosawa, Ichiro*; Igawa, Naoki
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 77(2), p.161 - 168, 2009/02
Times Cited Count:4 Percentile:10.14(Electrochemistry)We analyzed the mechanism of mixed conduction due to electrons and oxygen ions in (La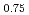 Sr
Sr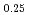 )MnO
)MnO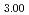 and (Ba
and (Ba Sr
Sr )(Co
)(Co Fe
Fe )O
)O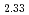 through the Rietveld refinement and maximum entropy method analyses of neutron and synchrotron X-ray diffractions. It was found that Mn-O plane in (La
through the Rietveld refinement and maximum entropy method analyses of neutron and synchrotron X-ray diffractions. It was found that Mn-O plane in (La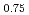 Sr
Sr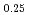 )MnO
)MnO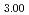 has a strong, isotropic covalent bond that enables conduction of electrons. The (Co, Fe)-O2 plane with a covalent bond and a low concentration of oxygen vacancies in (Ba
has a strong, isotropic covalent bond that enables conduction of electrons. The (Co, Fe)-O2 plane with a covalent bond and a low concentration of oxygen vacancies in (Ba Sr
Sr )(Co
)(Co Fe
Fe )O
)O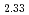 can also conduct electrons. On the other hand, the (Ba, Sr)-O1 plane in (Ba
can also conduct electrons. On the other hand, the (Ba, Sr)-O1 plane in (Ba Sr
Sr )(Co
)(Co Fe
Fe )O
)O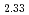 , which has a high concentration of oxygen vacancies, large isotropic displacement parameter, and a strong ionic bond, is responsible for the diffusion of oxygen ions.
, which has a high concentration of oxygen vacancies, large isotropic displacement parameter, and a strong ionic bond, is responsible for the diffusion of oxygen ions.
 Sr
Sr )(Co
)(Co Fe
Fe )O
)O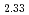 by Rietveld refinement and maximum entropy method analysis
by Rietveld refinement and maximum entropy method analysisIto, Takanori*; Nishida, Yuki*; Tomita, Aya*; Fujie, Yoshinori*; Kitamura, Naoto*; Idemoto, Yasushi*; Osaka, Keiichi*; Hirosawa, Ichiro*; Igawa, Naoki
Solid State Communications, 149(1-2), p.41 - 44, 2009/01
Times Cited Count:36 Percentile:76.35(Physics, Condensed Matter)The crystal structure and charge density of (Ba Sr
Sr )(Co
)(Co Fe
Fe )O
)O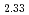 were investigated by the Rietveld refinement method and the maximum entropy method by using neutron and synchrotron X-ray diffraction. The crystal structure was refined by using the split atom model to cation sites with the space group,
were investigated by the Rietveld refinement method and the maximum entropy method by using neutron and synchrotron X-ray diffraction. The crystal structure was refined by using the split atom model to cation sites with the space group,  . The site occupancies of O1(4
. The site occupancies of O1(4 ) and O2(8
) and O2(8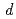 ) sites were 0.59 and 0.87, respectively. It was found that the (Co, Fe)-O2 plane in the sample has anisotropic covalent and ionic bands, and that the (Ba, Sr)-O1 bond was ionic with a low charge density.
) sites were 0.59 and 0.87, respectively. It was found that the (Co, Fe)-O2 plane in the sample has anisotropic covalent and ionic bands, and that the (Ba, Sr)-O1 bond was ionic with a low charge density.