Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 complexes of mixed N,O-donor ligands demonstrated on a 10-fold [Eu(TPAMEN)]
complexes of mixed N,O-donor ligands demonstrated on a 10-fold [Eu(TPAMEN)] chelate complex
chelate complexSchnaars, K.; Kaneko, Masashi; Fujisawa, Kiyoshi*
Inorganic Chemistry, 60(4), p.2477 - 2491, 2021/02
Times Cited Count:6 Percentile:59.03(Chemistry, Inorganic & Nuclear)To reduce high-level radiotoxic waste generated by nuclear power plants, highly selective separation agents for minor actinides are mandatory. The mixed N,O-donor ligand  -tetrakis[(6-carboxypyridin-2-yl)methyl]ethylenediamine (H
-tetrakis[(6-carboxypyridin-2-yl)methyl]ethylenediamine (H TPAEN) has shown good performance as a masking agent in Am
TPAEN) has shown good performance as a masking agent in Am /Eu
/Eu separation studies. In this work, we examine whether a decrease in O-donor basicity can promote the M
separation studies. In this work, we examine whether a decrease in O-donor basicity can promote the M -N
-N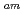 interactions. Therefore, we replace the deprotonated "charged" carboxylic acid groups of TPAEN
interactions. Therefore, we replace the deprotonated "charged" carboxylic acid groups of TPAEN by neutral amide groups and introduce
by neutral amide groups and introduce  -tetrakis[(6-
-tetrakis[(6-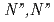 -diethylcarbamoylpyridin-2-yl)methyl]ethylenediamine (TPAMEN) as a new ligand. TPAMEN was crystallized with Eu(OTf)
-diethylcarbamoylpyridin-2-yl)methyl]ethylenediamine (TPAMEN) as a new ligand. TPAMEN was crystallized with Eu(OTf) and Eu(NO
and Eu(NO )
) 6H
6H O to form positively charged 1:1 [Eu(TPAMEN)]
O to form positively charged 1:1 [Eu(TPAMEN)] complexes in the solid state. Alterations in the M-O/N bond distances are compared to [Eu(TPAEN)]
complexes in the solid state. Alterations in the M-O/N bond distances are compared to [Eu(TPAEN)] and investigated by DFT calculations to expose the differences in charge/energy density distributions at europium(III) and the donor functionalities of the TPAEN
and investigated by DFT calculations to expose the differences in charge/energy density distributions at europium(III) and the donor functionalities of the TPAEN and TPAMEN. On the basis of estimations of the bond orders, atomic charges spin populations, and density of states in the Eu and potential Am and Cm complexes, the specific contributions of the donor-metal interaction are analyzed. The prediction of complex formation energy differences for the [M(TPAEN)]
and TPAMEN. On the basis of estimations of the bond orders, atomic charges spin populations, and density of states in the Eu and potential Am and Cm complexes, the specific contributions of the donor-metal interaction are analyzed. The prediction of complex formation energy differences for the [M(TPAEN)] and [M(TPAMEN)]
and [M(TPAMEN)] (M
(M = Eu
= Eu , Am
, Am ) complexes provide an outlook on the potential performance of TPAMEN in Am
) complexes provide an outlook on the potential performance of TPAMEN in Am /Eu
/Eu separation.
separation.
Shimojo, Kojiro; Fujiwara, Iori*; Fujisawa, Kiyoshi*; Okamura, Hiroyuki; Sugita, Tsuyoshi; Oshima, Tatsuya*; Baba, Yoshinari*; Naganawa, Hirochika
Solvent Extraction Research and Development, Japan, 23(2), p.151 - 159, 2016/05
Liquid-liquid extraction of rare-earth (RE) cations has been investigated using  -dodecyldiglycolamic acid (C
-dodecyldiglycolamic acid (C DGAA) with a secondary amide group, and compared with that using
DGAA) with a secondary amide group, and compared with that using  -dioctyldiglycolamic acid (DODGAA) with a tertiary amide group. C
-dioctyldiglycolamic acid (DODGAA) with a tertiary amide group. C DGAA enables quantitative transfer of all RE cations from moderately acidic solution, while being selective toward the heavier RE cations, and performs better than typical carboxylic-acid-type extractants. However, C
DGAA enables quantitative transfer of all RE cations from moderately acidic solution, while being selective toward the heavier RE cations, and performs better than typical carboxylic-acid-type extractants. However, C DGAA provides low extraction performance and separation ability for RE cations compared with DODGAA because of the weaker basicity of the amide oxygen. Slope analysis demonstrated that RE
DGAA provides low extraction performance and separation ability for RE cations compared with DODGAA because of the weaker basicity of the amide oxygen. Slope analysis demonstrated that RE transfer with C
transfer with C DGAA proceeded through a proton-exchange reaction, forming a 1:3 complex, RE(C
DGAA proceeded through a proton-exchange reaction, forming a 1:3 complex, RE(C DGAA)
DGAA) . Structural characterization by X-ray diffraction revealed that three
. Structural characterization by X-ray diffraction revealed that three  -butyldiglycolamic acid (C
-butyldiglycolamic acid (C DGAA) molecules coordinated to the La
DGAA) molecules coordinated to the La central ion in a tridentate fashion and the La
central ion in a tridentate fashion and the La primary coordination sphere consisted of three oxygen atoms from the amide group, three oxygen atoms from the ether group, and three oxygen atoms from the carboxy group.
primary coordination sphere consisted of three oxygen atoms from the amide group, three oxygen atoms from the ether group, and three oxygen atoms from the carboxy group.
*; *; Hitoki, Shigehisa*; Fujisawa, Noboru; Sugihara, Masayoshi; Yamamoto, Shin; Mizoguchi, Tadanori*; Abe, Mitsushi*; *; *; et al.
JAERI-M 87-119, 19 Pages, 1987/08
no abstracts in English
Abe, Mitsushi*; *; *; *; *; *; Shinya, K.*; Sugihara, Masayoshi; Hatayama, Akiyoshi*; Hitoki, Shigehisa*; et al.
JAERI-M 87-116, 13 Pages, 1987/08
no abstracts in English
*; *; Sugihara, Masayoshi; Yamamoto, Shin; Mizoguchi, Tadanori*; Hitoki, Shigehisa*; Abe, Mitsushi*; *; *; *; et al.
JAERI-M 87-110, 11 Pages, 1987/08
no abstracts in English
Hitoki, Shigehisa*; Sugihara, Masayoshi; Yamamoto, Shin; Abe, Mitsushi*; *; *; *; *; Shinya, K.*; Hatayama, Akiyoshi*; et al.
JAERI-M 87-109, 33 Pages, 1987/08
no abstracts in English
Sugihara, Masayoshi; Mizoguchi, Tadanori*; Hatayama, Akiyoshi*; Shinya, K.*; Yamamoto, Shin; Abe, Mitsushi*; *; *; *; *; et al.
JAERI-M 87-108, 49 Pages, 1987/08
no abstracts in English
Fujisawa, Noboru; Sugihara, Masayoshi; Yamamoto, Shin; Mizoguchi, Tadanori*; Hitoki, Shigehisa*; Abe, Mitsushi*; *; *; *; *; et al.
JAERI-M 87-093, 64 Pages, 1987/07
no abstracts in English
 -ray irradiation in zeolite-water mixture
-ray irradiation in zeolite-water mixtureSugawara, Atsushi*; Kumagai, Yuta; Watanabe, Masayuki; Kimura, Atsushi*; Taguchi, Mitsumasa*; Fujisawa, Kiyoshi*
no journal, ,
Radiation-induced decomposition of three chlorinated aromatic compounds in zeolite-water mixture was studied, in order to evaluate decomposition yields of the aromatics adsorbed on the zeolite. The adsorption and condensation in zeolite pore structure has a possibility to improve the decomposition yield of aqueous organics in treatments of environmental water using ionizing radiations. In this study, the decomposition yields of 4-chlorophenol (4-ClPh), 4-chlorobenzoic acid (4-ClBAc), 4-chlorobenzylamine(4-ClBAm) were examined. The decomposition yield of adsorbed 4-ClBAm was lower than that in pure water. On the other hand, adsorbed 4-ClPh decomposed at a yield as high as in pure water. Moreover, a slight increase in the yield was overserved for 4-ClBAc. These results suggest that the decomposition yields of adsorbed aromatics are dependent on the reactivity with carriers of energy transfer from solid zeolite framework to water in the pore.
Murayama, Tatsuya*; Watanabe, Masayuki; Fujisawa, Kiyoshi*
no journal, ,
In this presentation, N,N,N',N'-Tetrakis(2-pyridylmethyl)ethylenediamine(TPEN) and the derivative, N,N,N',N'-Tetrakis(2-pyridylmethyl)-1,2-propanediamine(MeTPEN) which methylene chain was substituted by methyl group were used as ligand to synthesize europium complexes and which molecular structures and properties were compared by using spectroscopic methods.
Murayama, Tatsuya*; Watanabe, Masayuki; Aoyagi, Noboru; Fujisawa, Kiyoshi*
no journal, ,
In this study, we synthesized Eu(III) complex using N,N,N',N'-tetrakis(2-pyridylmethyl)-ethylenediamine (TPEN) and TPEN derivatives, N,N,N',N'-tetrakis(2-pyridylmethyl)-propylenediamine(MeTPEN) which has methyl group into the ethylenediamine framework, the structure and physical properties of these complexes investigated. The crystal structure of Eu(TPEN) and Eu(MeTPEN), the geometry of these structures indicate pseudo-bicapped square antiprism (10 coordination) with two bidentate NO
 ; ions. As the result of investigation of fluorescence spectrum and lifetime in organic solution, the fluorescence of Eu (MeTPEN) was enhanced more intensity than that of Eu(TPEN) by addition of OAc
; ions. As the result of investigation of fluorescence spectrum and lifetime in organic solution, the fluorescence of Eu (MeTPEN) was enhanced more intensity than that of Eu(TPEN) by addition of OAc ; ion.
; ion.