Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Fujiwara, Asako; Hoshi, Akiko; Kameo, Yutaka; Nakashima, Mikio
Journal of Chromatography A, 1216(18), p.4125 - 4127, 2009/03
Times Cited Count:10 Percentile:30.73(Biochemical Research Methods)Dependence of Th recovery on HF concentration in nitric acid solutions (1 5 mol/dm
5 mol/dm ) containing 1
) containing 1 10
10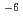 mol/dm
mol/dm of Th and various concentrations of HF was studied using a commercially available UTEVA resin column (for uranium and tetravalent actinide). Thorium recovery decreased with an increase in the HF concentration in the sample solutions. The concentration of HF at which Th recovery started to decrease was about 1
of Th and various concentrations of HF was studied using a commercially available UTEVA resin column (for uranium and tetravalent actinide). Thorium recovery decreased with an increase in the HF concentration in the sample solutions. The concentration of HF at which Th recovery started to decrease was about 1 10
10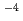 mol/dm
mol/dm in 1 mol/dm
in 1 mol/dm HNO
HNO solution, about 1
solution, about 1 10
10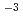 mol/dm
mol/dm in 3 mol/dm
in 3 mol/dm HNO
HNO solution, and about 1
solution, and about 1 10
10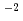 mol/dm
mol/dm in 5 mol/dm
in 5 mol/dm HNO
HNO solution. When Al(NO
solution. When Al(NO )
) (0.2 mol/dm
(0.2 mol/dm ) or Fe(NO
) or Fe(NO )
) (0.6 mol/dm
(0.6 mol/dm ) was added as a masking agent for F
) was added as a masking agent for F into the Th solution containing 1
into the Th solution containing 1 10
10 mol/dm
mol/dm HF and 1 mol/dm
HF and 1 mol/dm HNO
HNO , the Th recovery improved from 1.4
, the Th recovery improved from 1.4 0.3% to 95
0.3% to 95 5% or 93
5% or 93 3%. Effective extraction of Th on UTEVA resin was achieved by selecting the concentration of HNO
3%. Effective extraction of Th on UTEVA resin was achieved by selecting the concentration of HNO and/or adding masking agents such as Al(NO
and/or adding masking agents such as Al(NO )
) according to the concentration of HF in the sample solution.
according to the concentration of HF in the sample solution.
Hoshi, Akiko; Watanabe, Koichi; Fujiwara, Asako; Haraga, Tomoko; Kameo, Yutaka; Nakashima, Mikio; Takebe, Shinichi
Nihon Genshiryoku Gakkai Wabun Rombunshi, 7(3), p.177 - 185, 2008/09
The simple and rapid separation method was developed for actinides in the low-level radioactive waste. Extraction chromatographic columns were used for the separation of U, Np, Pu, Am, and Cm in the solution of the simulated solidified product and the simulated waste solution. In the investigation of separation procedure, it was tried to construct the scheme with the relatively non-corrosive reagents aiming to apply to the routine analysis of the radioactive waste. Recoveries and decontamination factors of actinides in the solution of simulated waste were high enough to determine of actinides in radioactive waste by alpha-spectrometry, mass spectroscopy. The time required of the separation operation was 2-3 hours. The chromatographic method was applied to analysis of actinide in actual waste solution, high recoveries and decontamination factors were obtained, which indicated that the extraction chromatographic separation method would be adopted as a simple and rapid separation method of actinide in waste.
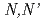 -dimethyl-
-dimethyl-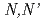 -diphenylpyridine-2,6-dicarboxyamide complexes
-diphenylpyridine-2,6-dicarboxyamide complexesFujiwara, Asako; Nakano, Yoshiharu*; Yaita, Tsuyoshi; Okuno, Kenji*
Journal of Alloys and Compounds, 456(1-2), p.429 - 435, 2008/05
Times Cited Count:17 Percentile:63.04(Chemistry, Physical)The tridentate ligand 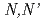 -dimethyl-
-dimethyl-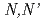 -diphenylpyridine-2,6-dicarboxyamide (DMDPhPDA) and the corresponding lanthanum complex [La(NO
-diphenylpyridine-2,6-dicarboxyamide (DMDPhPDA) and the corresponding lanthanum complex [La(NO )
) (DMDPhPDA)
(DMDPhPDA) ] have been prepared and structurally characterised. In the lanthanum complex, two DMDPhPDA molecules coordinated to La(III) in a tridentate fashion and to three nitrate ions in a bidentate fashion make the lanthanum atom 12-coordinate. The stability constants determined by spectrophotometric titration suggest that [Ln(DMDPhPDA)
] have been prepared and structurally characterised. In the lanthanum complex, two DMDPhPDA molecules coordinated to La(III) in a tridentate fashion and to three nitrate ions in a bidentate fashion make the lanthanum atom 12-coordinate. The stability constants determined by spectrophotometric titration suggest that [Ln(DMDPhPDA) ]
] is the primary product in nitrate media and [Ln(DMDPhPDA)
is the primary product in nitrate media and [Ln(DMDPhPDA) ]
] is difficult to form. However, [Ln(DMDPhPDA)
is difficult to form. However, [Ln(DMDPhPDA) ]
] could not be distinguished in
could not be distinguished in 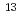 C NMR spectra. The
C NMR spectra. The 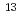 C NMR titration results imply that a fast ligand exchange process takes place.
C NMR titration results imply that a fast ligand exchange process takes place.
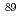 Sr and
Sr and  Sr in radioactive waste using Sr extraction disk and beta-ray spectrometer
Sr in radioactive waste using Sr extraction disk and beta-ray spectrometerKameo, Yutaka; Katayama, Atsushi; Fujiwara, Asako; Haraga, Tomoko; Nakashima, Mikio
Journal of Radioanalytical and Nuclear Chemistry, 274(1), p.71 - 78, 2007/10
Times Cited Count:21 Percentile:78.10(Chemistry, Analytical)no abstracts in English
Fujiwara, Asako; Kameo, Yutaka; Katayama, Atsushi; Nakashima, Mikio
Nihon Genshiryoku Gakkai Wabun Rombunshi, 6(1), p.58 - 64, 2007/03
no abstracts in English
Fujiwara, Asako; Kameo, Yutaka; Hoshi, Akiko; Haraga, Tomoko; Nakashima, Mikio
Journal of Chromatography A, 1140(1-2), p.163 - 167, 2007/01
Times Cited Count:25 Percentile:57.80(Biochemical Research Methods)Extraction chromatography with UTEVA resin was applied to separation of Th and U from control solutions prepared from a multi-element control solution and from sample solutions of solidified simulated waste. Thorium and U in control solutions with 1 to 5 M HNO were extracted with UTEVA resin and recovered with a solution containing 0.1 M HNO
were extracted with UTEVA resin and recovered with a solution containing 0.1 M HNO and 0.05 M oxalic acid to be separated from the other metallic elements. Extraction behavior of U in the sample solutions was similar to that in the control solutions, but extraction of Th was dependent on the concentration of HNO
and 0.05 M oxalic acid to be separated from the other metallic elements. Extraction behavior of U in the sample solutions was similar to that in the control solutions, but extraction of Th was dependent on the concentration of HNO . Thorium was extracted from 5 M HNO
. Thorium was extracted from 5 M HNO sample solutions but not from 1 M HNO
sample solutions but not from 1 M HNO sample solutions. We conjecture that thorium fluoride formation interferes with extraction of Th. Addition of Al(NO
sample solutions. We conjecture that thorium fluoride formation interferes with extraction of Th. Addition of Al(NO )
) and Fe(NO
and Fe(NO )
) , which have a higher stability constant with fluoride ion than Th does with it improved extractability of Th from 1 M HNO
, which have a higher stability constant with fluoride ion than Th does with it improved extractability of Th from 1 M HNO sample solution.
sample solution.
Kameo, Yutaka; Fujiwara, Asako; Watanabe, Koichi; Kono, Nobuaki; Nakashima, Mikio
Nihon Genshiryoku Gakkai Wabun Rombunshi, 4(3), p.187 - 193, 2005/09
no abstracts in English
Fujiwara, Asako; Kameo, Yutaka; Haraga, Tomoko; Nakashima, Mikio
no journal, ,
no abstracts in English
 on Th extraction with UTEVA resin
on Th extraction with UTEVA resinFujiwara, Asako; Kameo, Yutaka; Nakashima, Mikio
no journal, ,
no abstracts in English
Fujiwara, Asako; Kameo, Yutaka; Nakashima, Mikio
no journal, ,
Separation of Th and U in dissolved solution of solidified products of simulated waste with UTEVA resin has been investigated. Thorium and U were separated from the other elements such as Na, Al, Ca, Fe by column chromatography with UTEVA resin and recovered with the solution containing 0.1 M HNO and 0.05 M oxalic acid. The concentration of Am and Pu in the fraction to recover Th and U was under the detection limit. Although 1% of Np was contaminated to the recovery fraction of Th and U, Np would not interfere the measurement of U. UTEVA resin cartridge system with flow pomp was applied to the separation of Th and U. The result from cartridge system showed good separation of Th and U from Na, Al, Ca, and Fe. The flow rate with cartridge system was 4-5 times faster than that with column system to shorten the operation time.
and 0.05 M oxalic acid. The concentration of Am and Pu in the fraction to recover Th and U was under the detection limit. Although 1% of Np was contaminated to the recovery fraction of Th and U, Np would not interfere the measurement of U. UTEVA resin cartridge system with flow pomp was applied to the separation of Th and U. The result from cartridge system showed good separation of Th and U from Na, Al, Ca, and Fe. The flow rate with cartridge system was 4-5 times faster than that with column system to shorten the operation time.