Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yamashita, Kiyoto; Maki, Shota; Yokosuka, Kazuhiro; Fukui, Masahiro; Iemura, Keisuke
JAEA-Technology 2023-023, 97 Pages, 2024/03
The incinerator adopted to incineration room, Plutonium Waste Treatment Facility had been demonstrated since 2002 for developing technologies to reduce the volume of fire-resistant wastes such as vinyl chloride (represented by Polyvinyl chloride bags) and rubber gloves for Radio Isotope among radioactive solid wastes generated by the production of mixed oxide fuels. The incinerator, cooling tower, and processing pipes were replaced with a suspension period from 2018 to 2022, which fireproof materials on the inner wall of the incinerator was cracked and grown caused by hydrogen chloride generated when disposing of fire-resistant wastes. This facility consists of the waste feed process, the incineration process, the waste gas treatment process, and the ash removal process. We replaced the cooling tower in the waste gas treatment process from March 2020 to March 2021, and the incinerator in the incineration process from January 2021 to February 2022. In addition, samples were collected from the incinerator and the cooling tower during the removing and dismantling of the replaced devices, observed by Scanning Electron Microscope and X-ray microanalyzer, and analyzed by X-ray diffraction to investigate the corrosion and deterioration of them. This report describes the method of setting up the green house, the procedure for replacing them, and the results from analysis in corrosion and deterioration of the cooling tower and incinerator.
Yamashita, Kiyoto; Yokoyama, Aya*; Takagai, Yoshitaka*; Maki, Shota; Yokosuka, Kazuhiro; Fukui, Masahiro; Iemura, Keisuke
JAEA-Technology 2022-020, 106 Pages, 2022/10
Radioactive solid wastes generated by Fukushima Daiichi Nuclear Power Station disaster may contain high levels of salt from the tsunami and seawater deliberately released into the area. It is assumed that polyvinyl chloride (PVC) products may be used for decommissioning work and for containment of radioactive wastes in the future. Among the method of handling them, incineration is one method that needs to be investigated as it is good method for reduction and stabilization of wastes. But in order to dispose of Trans-Uranic (TRU) solid waste containing chlorides, it is necessary to select the structure and materials of the facility based on the information such as the movement of nuclides and chlorides in the waste gas treating system and the corrosion of equipment due to chlorides. Therefore, we decided to get various data necessary to design a study of the incineration facilities. And we decided to examine the transfer behavior of chlorides to the waste gas treatment system, the corrosion-resistance of materials in the incineration facilities, and the distribution survey of plutonium in them obtained using the Plutonium-contaminated Waste Treatment Facility (PWTF), Nuclear Fuel Cycle Engineering Laboratories, which is a unique incinerating facility in Japan. This report describes the transfer behavior of chlorides in the waste gas treatment system, the evaluation of corrosion-resistance materials and the distribution survey of plutonium in the incineration facilities obtained by these tests using the Plutonium-contaminated Waste Treatment Facility, Nuclear Fuel Cycle Engineering Laboratories.
Segawa, Tomoomi; Kawaguchi, Koichi; Ishii, Katsunori; Suzuki, Masahiro; Fukasawa, Tomonori*; Fukui, Kunihiro*
Funtai Kogakkai-Shi, 57(9), p.485 - 494, 2020/09
In the spent fuel reprocessing process, a mixed solution of uranyl nitrate and plutonium nitrate is converted into mixed oxide powder by the microwave heating. To evaluate the applicability to the industrial-scale and acquire the characteristics data of the microwave heating denitration of various metal nitrate aqueous solutions based on the knowledge studied in the development of laboratory-scale basic experiments, the microwave heating characteristics and metal oxide powder properties were investigated using cerium nitrate, cobalt nitrate and copper nitrate aqueous solutions. The progress rate of the denitration reaction was depended on the position, and the denitration reaction proceeded faster at the periphery than at the center. The morphologies of the synthesized products were porous and hard dry solid with cerium nitrate aqueous solution, foamed dry solid with cobalt nitrate aqueous solution, and powdery particles with copper nitrate aqueous solution. The denitration ratio and average particle size of the synthesized products increased in the order of the cerium nitrate aqueous solution, the cobalt nitrate aqueous solution, and the copper nitrate aqueous solution. The numerical simulations revealed that the periphery of the bottom surface of the metal nitrate aqueous solution was heated by microwaves. This results consistent with the experimental results in which the denitration reaction started from the periphery of the metal nitrate aqueous solution.
Segawa, Tomoomi; Fukasawa, Tomonori*; Huang, A.-N.*; Yamada, Yoshikazu; Suzuki, Masahiro; Fukui, Kunihiro*
Chemical Engineering Science, 153, p.108 - 116, 2016/10
Times Cited Count:7 Percentile:23.93(Engineering, Chemical)The influence of the heating method and rate on the morphology of CuO powders synthesized from Cu(NO )
)
 3H
3H O aqueous solutions by denitration was investigated. The median diameter of the obtained powder was found to decrease as the heating rate increased, independent of the heating method. The microwave heating method remarkably reduced the particle size and enhanced the irregularity and disorder of the shape and surface of the particles, which were found to be more widely distributed. In contrast, the microwave hybrid heating method yielded the most spherical particles with the smoothest surface. It was also found that this heating method sharpened the particle size distribution and had higher energy efficiency than the MW method. Numerical simulations also indicated a difference in the energy efficiency between these two methods. The simulations also revealed that the hybrid method could heat the whole reactor more uniformly with a lower microwave output.
O aqueous solutions by denitration was investigated. The median diameter of the obtained powder was found to decrease as the heating rate increased, independent of the heating method. The microwave heating method remarkably reduced the particle size and enhanced the irregularity and disorder of the shape and surface of the particles, which were found to be more widely distributed. In contrast, the microwave hybrid heating method yielded the most spherical particles with the smoothest surface. It was also found that this heating method sharpened the particle size distribution and had higher energy efficiency than the MW method. Numerical simulations also indicated a difference in the energy efficiency between these two methods. The simulations also revealed that the hybrid method could heat the whole reactor more uniformly with a lower microwave output.
Segawa, Tomoomi; Fukasawa, Tomonori*; Yamada, Yoshikazu; Suzuki, Masahiro; Yoshida, Hideto*; Fukui, Kunihiro*
Proceedings of Asian Pacific Confederation of Chemical Engineering 2015 (APCChE 2015), 8 Pages, 2015/09
A mixed solution of uranyl nitrate and plutonium nitrate is converted to MOX raw powder by the microwave heating de-nitration method in nuclear reprocessing. Copper oxide synthesized by heating de-nitration was used as a model for the de-nitration process. The microwave heating method (MW) and infrared heating method (IR) were used, and how they and their heating rate influence the obtained particle morphology and size were investigated. The particles obtained by the MW and IR were sufficiently similar in the surface morphology and the mass median diameter was decreased by the increased heating rate. The mass median diameters by the MW were the heating rate and smaller than those obtained by IR. The particle size distribution of the particle obtained by the MW was broader than that by the IR. The relationship of the temperature distribution and particle size distribution by the MW was discussed by the numerical simulation.
Segawa, Tomoomi; Kawaguchi, Koichi; Ishii, Katsunori; Suzuki, Masahiro; Arimitsu, Naoki*; Yoshida, Hideto*; Fukui, Kunihiro*
Advanced Powder Technology, 26(3), p.983 - 990, 2015/05
Times Cited Count:8 Percentile:25.46(Engineering, Chemical)Denitration of the aqueous solution of nickel nitrate hexahydrate (Ni(NO )
)
 6H
6H O) by a microwave heating method was investigated. Since Ni(NO
O) by a microwave heating method was investigated. Since Ni(NO )
)
 6H
6H O aqueous solution cannot be heated to over 300
O aqueous solution cannot be heated to over 300  C by microwave irradiation owing to the low microwave absorptivity of its intermediate, NiO could not previously be obtained by microwave heating. We propose a novel NiO synthesis method that uses microwave heating without the risk of chemical contamination. A NiO powder reagent was added to the solution as a microwave acceptor. The denitration efficiency to NiO could be improved by an adiabator around the reactor to increase the temperature homogeneity in the reactor. Numerical simulations also reveal that the use of the adiabator results in remarkable changes in the electromagnetic field distribution in the reactor, temperature inhomogeneity decreases.
C by microwave irradiation owing to the low microwave absorptivity of its intermediate, NiO could not previously be obtained by microwave heating. We propose a novel NiO synthesis method that uses microwave heating without the risk of chemical contamination. A NiO powder reagent was added to the solution as a microwave acceptor. The denitration efficiency to NiO could be improved by an adiabator around the reactor to increase the temperature homogeneity in the reactor. Numerical simulations also reveal that the use of the adiabator results in remarkable changes in the electromagnetic field distribution in the reactor, temperature inhomogeneity decreases.
Fukui, Kunihiro*; Igawa, Yusuke*; Arimitsu, Naoki*; Suzuki, Masahiro; Segawa, Tomoomi; Fujii, Kanichi*; Yamamoto, Tetsuya*; Yoshida, Hideto*
Chemical Engineering Journal, 211-212, p.1 - 8, 2012/11
Times Cited Count:13 Percentile:39.33(Engineering, Environmental)The process for synthesizing metallic oxide powders by the microwave denitration method was investigated using hexahydrated nickel nitrate and trihydrated copper nitrate aqueous solutions, and the electrical field and the temperature distributions in the reactor were numerically simulated. Although CuO powder can be obtained from a trihydrated copper nitrate aqueous solution by the microwave denitration method, a hexahydrated nickel nitrate aqueous solution cannot be heated up to over 270  C by microwave irradiation. It was also found that the reaction routes for microwave heating are the same as those for conventional external heating. This finding indicates that the success of producing oxide particles by microwave denitration depends not only on the microwave absorptivity of the intermediate and the metallic oxide, but also on the temperature difference.
C by microwave irradiation. It was also found that the reaction routes for microwave heating are the same as those for conventional external heating. This finding indicates that the success of producing oxide particles by microwave denitration depends not only on the microwave absorptivity of the intermediate and the metallic oxide, but also on the temperature difference.
Iwamoto, Hiroki; Imamura, Minoru*; Koba, Yusuke*; Fukui, Yoshinori*; Wakabayashi, Genichiro*; Uozumi, Yusuke*; Kin, Tadahiro; Iwamoto, Yosuke; Hohara, Shinya*; Nakano, Masahiro*
Physical Review C, 82(3), p.034604_1 - 034604_8, 2010/09
Times Cited Count:13 Percentile:62.02(Physics, Nuclear)We investigate proton-production double-differential cross sections (DDXs) for 300- and 392-MeV proton-induced reactions on O, V, Tb, Ta, Au, Pb, and Bi. Emitted proton energies are measured with stacked scintillator spectrometers by the 
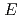 -
-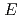 technique. Experimental results are compared with the intranuclear cascade (INC) and quantum molecular dynamics models. Although both models can reproduce spectral DDXs, there is a difference at the most forward and backward angles. The cause of these differences is discussed in terms of the refraction caused by the nuclear potential. Angular distributions of the present data are well accounted for by the Kalbach systematics plus INC one-step calculations. The quasi-free-scattering contribution increases with decreasing target mass and increasing emission energy.
technique. Experimental results are compared with the intranuclear cascade (INC) and quantum molecular dynamics models. Although both models can reproduce spectral DDXs, there is a difference at the most forward and backward angles. The cause of these differences is discussed in terms of the refraction caused by the nuclear potential. Angular distributions of the present data are well accounted for by the Kalbach systematics plus INC one-step calculations. The quasi-free-scattering contribution increases with decreasing target mass and increasing emission energy.
Shibata, Yuichi; Hareo, Ueta,; Sato, Shunichi; Fukui, Masahiro; Gorai, Hiroyasu*; Tamura, Tetsuro*
Dekomisshoningu Giho, (29), p.2 - 12, 2004/03
None
Sawada, Sho; Maki, Shota; Yokosuka, Kazuhiro; Fukui, Masahiro; Iemura, Keisuke; Osawa, Takayasu
no journal, ,
no abstracts in English
Fukui, Kunihiro*; Arimitsu, Naoki*; Yamamoto, Tetsuya*; Yoshida, Hideto*; Yamamoto, Takuma*; Ishii, Katsunori; Suzuki, Masahiro
no journal, ,
no abstracts in English
Saiki, Yuta*; Fukui, Kunihiro*; Yamamoto, Tetsuya*; Yoshida, Hideto*; Yamamoto, Takuma*; Ishii, Katsunori; Suzuki, Masahiro
no journal, ,
no abstracts in English
Segawa, Tomoomi; Suzuki, Masahiro; Fujii, Kanichi; Igawa, Yusuke*; Arimitsu, Naoki*; Yamamoto, Tetsuya*; Fukui, Kunihiro*; Yoshida, Hideto*
no journal, ,
The synthesis process of the denitration reaction by the microwave heating method was investigated using a nickel nitrate (Ni(NO )
) ) aqueous solution. NiO powder cannot be obtained from Ni(NO
) aqueous solution. NiO powder cannot be obtained from Ni(NO )
) aqueous solution by the microwave heating method because of low microwave absorption of the intermediate obtained from nitrate aqueous solution. In the present work, it was showed that Ni(NO
aqueous solution by the microwave heating method because of low microwave absorption of the intermediate obtained from nitrate aqueous solution. In the present work, it was showed that Ni(NO )
) aqueous solution with 6.0 g of NiO powder as a microwave absorber can be completely converted to NiO powder by microwave heating. NiO powder promotes the denitration reaction, and reduces the required reaction time with increasing the amount of NiO powder. Furthermore the adiabator was set around the reactor in order to equalize the temperature distribution in the reactor. It was also found that the denitration efficiency of Ni(NO
aqueous solution with 6.0 g of NiO powder as a microwave absorber can be completely converted to NiO powder by microwave heating. NiO powder promotes the denitration reaction, and reduces the required reaction time with increasing the amount of NiO powder. Furthermore the adiabator was set around the reactor in order to equalize the temperature distribution in the reactor. It was also found that the denitration efficiency of Ni(NO )
) aqueous solution to NiO powder can be improved by using the adiabator when the center of the reactor has the same temperature.
aqueous solution to NiO powder can be improved by using the adiabator when the center of the reactor has the same temperature.
Maki, Shota; Shibata, Yuichi; Yokosuka, Kazuhiro; Fukui, Masahiro; Iemura, Keisuke; Osawa, Takayasu
no journal, ,
no abstracts in English
Maki, Shota; Fukui, Masahiro; Iemura, Keisuke; Osawa, Takayasu
no journal, ,
no abstracts in English
Yokosuka, Kazuhiro; Maki, Shota; Fukui, Masahiro; Iemura, Keisuke; Osawa, Takayasu
no journal, ,
no abstracts in English
Maki, Shota; Yokosuka, Kazuhiro; Fukui, Masahiro; Iemura, Keisuke; Osawa, Takayasu
no journal, ,
no abstracts in English
Segawa, Tomoomi; Kawaguchi, Koichi; Ishii, Katsunori; Suzuki, Masahiro; Fukasawa, Tomonori*; Fukui, Kunihiro*
no journal, ,
In the reprocessing process of spent fuels, a mixed solution of uranyl nitrate and plutonium nitrate is converted into mixed oxide powders by the microwave heating. To evaluate the applicability to the macro-scale based on the knowledge obtained in the laboratory-scale experiments, the microwave heating characteristics and metal oxide powder morphologies using a cerium nitrate, copper nitrate and cobalt nitrate aqueous solution were investigated. It was revealed that the denitration rates and the average particle sizes of the products increased in the order of the cerium nitrate aqueous solution, cobalt nitrate aqueous solution, and copper nitrate aqueous solution, and the denitration rate tends to increase at the periphery position and the average particle size at the center position.
Fukui, Masahiro; Yokosuka, Kazuhiro; Maki, Shota; Shibata, Yuichi; Shigihara, Yuta; Ouchi, Takahiro; Minouchi, Hiroyuki; Iemura, Keisuke
no journal, ,
no abstracts in English
Maki, Shota; Yokosuka, Kazuhiro; Shibata, Yuichi; Fukui, Masahiro; Iemura, Keisuke
no journal, ,
no abstracts in English