Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tanaka, Kazuya; Takahashi, Yoshio*; Fukushi, Keisuke*; Utsunomiya, Satoshi*
Chikyu Kagaku, 49(4), p.169 - 171, 2015/12
Many studies have been carried out since the Fukushima Dai-ichi Nuclear Power Plant (FDNPP) accident. This paper introduces the special issue on the FDNPP accident which includes contributions from various fields covering aerosols, soil, forest, river, ocean and application of new analytical technique to samples collected in Fukushima.
Fukushi, Keisuke*; Hasegawa, Yusuke*; Maeda, Koshi*; Aoi, Yusuke*; Tamura, Akihiro*; Arai, Shoji*; Yamamoto, Yuhei*; Aosai, Daisuke*; Mizuno, Takashi
Environmental Science & Technology, 47(22), p.12811 - 12818, 2013/11
Times Cited Count:27 Percentile:58.19(Engineering, Environmental)Eu(III) sorption on granite was examined by the combined microscopic and macroscopic approaches. Polished thin sections of the granite were reacted with solutions containing 10  M of Eu(III) and analyzed using EPMA and LA-ICP-MS. The Eu enrichment up to 6 wt.% was observed on most of the biotite grains. The Eu-enriched parts commonly lose K, which is the interlayer cation of biotite, indicating that the sorption mode is cation exchange in the interlayer. Batch Eu(III) sorption experiments on granite and biotite powders were conducted. The macroscopic sorption behavior of biotite was consistent with that of granite. The obtained sorption edges can be reproduced reasonably by the modeling considering single-site cation exchange reactions. Granite is complex mineral assemblages. However, the combined microscopic and macroscopic approaches revealed that elementary reactions by single phase can be representative for the bulk sorption reaction in complex mineral assemblages.
M of Eu(III) and analyzed using EPMA and LA-ICP-MS. The Eu enrichment up to 6 wt.% was observed on most of the biotite grains. The Eu-enriched parts commonly lose K, which is the interlayer cation of biotite, indicating that the sorption mode is cation exchange in the interlayer. Batch Eu(III) sorption experiments on granite and biotite powders were conducted. The macroscopic sorption behavior of biotite was consistent with that of granite. The obtained sorption edges can be reproduced reasonably by the modeling considering single-site cation exchange reactions. Granite is complex mineral assemblages. However, the combined microscopic and macroscopic approaches revealed that elementary reactions by single phase can be representative for the bulk sorption reaction in complex mineral assemblages.
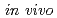 counting to organ doses in FDG-PET
counting to organ doses in FDG-PETTsuda, Keisuke; Kinase, Sakae; Fukushi, Masahiro*; Saito, Kimiaki
Hoken Butsuri, 42(4), p.349 - 352, 2007/12
no abstracts in English
Manaka, Mitsuo*; Yanase, Nobuyuki; Sato, Tsutomu*; Fukushi, Keisuke*
Geochemical Journal, 41(1), p.17 - 27, 2007/00
Times Cited Count:29 Percentile:56.2(Geochemistry & Geophysics)Natural attenuation was investigated for the antimony in the drainage water of an abandoned mine. Drainage water, waste rocks, and ocherous precipitates collected from the mine were investigated in terms of their mineralogy and chemistry. The chemistry of the drainage water was analyzed by measuring pH, ORP, and EC on site as well as by ICP-MS and ion chromatography. The results of these investigations indicated that Sb, which is generated by the dissolution of stibnite (Sb S
S ) and secondary formed Sb minerals in waste rocks, was attenuated by iron-bearing ocherous precipitates, especially schwertmannite, that form over time in the drainage water. Bulk distribution coefficients (Kd) for this Sb adsorption to the precipitates ranges up to at least 10
) and secondary formed Sb minerals in waste rocks, was attenuated by iron-bearing ocherous precipitates, especially schwertmannite, that form over time in the drainage water. Bulk distribution coefficients (Kd) for this Sb adsorption to the precipitates ranges up to at least 10 L/kg.
L/kg.
Tsuda, Keisuke; Kinase, Sakae; Fukushi, Masahiro*; Saito, Kimiaki
KEK Proceedings 2006-4, p.88 - 93, 2006/11
no abstracts in English
Fukushi, Keisuke*; Sato, Tsutomu*; Yanase, Nobuyuki; Minato, Junichi*; Yamada, Hirohisa*
American Mineralogist, 89(11-12), p.1728 - 1734, 2004/11
The sorption mechanism of As(V) on schwertmannite was investigated by both a batch sorption experiment and crystallographic considerations. The batch experiment was carried out as a function of As(V) concentration in acidic solution at 25  C. Crystallographic considerations indicate surface sites of schwertmannite comprise varied surface oxygen (hydroxyl) and SO
C. Crystallographic considerations indicate surface sites of schwertmannite comprise varied surface oxygen (hydroxyl) and SO groups. Sorption experiments showed reactive surface sites for As(V) sorption are surface SO
groups. Sorption experiments showed reactive surface sites for As(V) sorption are surface SO groups. As(V) sorption mechanism involves ligand exchange with solid phase SO
groups. As(V) sorption mechanism involves ligand exchange with solid phase SO . Results also suggest monodentate As(V) coordination with surface adsorbed SO
. Results also suggest monodentate As(V) coordination with surface adsorbed SO sites and bidentate As(V) coordination in structural originated SO
sites and bidentate As(V) coordination in structural originated SO sites. Estimated equilibrium constant for ligand exchange reaction describes the observed As(V) sorption behavior. The surface structure approach in this study reveals reactive surface sites in As(V) sorption on schwertmannite comprise surface SO
sites. Estimated equilibrium constant for ligand exchange reaction describes the observed As(V) sorption behavior. The surface structure approach in this study reveals reactive surface sites in As(V) sorption on schwertmannite comprise surface SO group instead of surface hydroxyl groups identified by former views.
group instead of surface hydroxyl groups identified by former views.
Fukushi, Keisuke*; Sasaki, Miwa*; Sato, Tsutomu*; Yanase, Nobuyuki; Amano, Hikaru; Ikeda, Hodaka*
Applied Geochemistry, 18(8), p.1267 - 1278, 2003/08
Times Cited Count:220 Percentile:95.9(Geochemistry & Geophysics)At Nishinomaki abandoned mine district, the water is acidic and contains much amounts of arsenic. However, arsenic concentration decreases downward without any artificial treatment. To understand the mechanism of the natural attenuation, the acid mine drainage and the ochreous precipitates were collected. The samples were analyzed by XRD, IR, ICP-MS and ion-chromatograph. The precipitates were investigated by selective extraction procedure. These results were interpreted with those calculated by the geochemical code. The contamination of water has been result from oxidation of pyrite and realgar and subsequent release of iron. The released ferrous iron transforms to ferric form by bacterial oxidation and then schwertmannite forms immediately. While the arsenic concentrations in the stream are lowered to background level at downstream, these in the ochreous precipitates are up to 60 mg/g. The iron hydroxide has been known to exhibit the high sorption affinity to arsenate. Hence, arsenic is effectively removed by the schwertmannite from the contaminated water and attenuated naturally.
Fukushi, Keisuke*; Sato, Tsutomu*; Yanase, Nobuyuki
Environmental Science & Technology, 37(16), p.3581 - 3586, 2003/07
Times Cited Count:71 Percentile:79.91(Engineering, Environmental)The mechanism of As(V) sorption on schwertmannite was investigated by a batch sorption experiment as a function of solution As(V) concentration under acidic conditions (pH 3.3  0.1) at 25
0.1) at 25  C. The reacted solution chemistry and mineralogy showed that the mechanism of As(V) sorption was ligand exchange with solid phase SO
C. The reacted solution chemistry and mineralogy showed that the mechanism of As(V) sorption was ligand exchange with solid phase SO in schwertmannite. Two processes presumably occur simultaneously within the reaction period. i.e., ligand exchange of As(V) with surface site SO
in schwertmannite. Two processes presumably occur simultaneously within the reaction period. i.e., ligand exchange of As(V) with surface site SO and subsequent transfer of As(V) to the structure and ligand exchange with tunnel site SO
and subsequent transfer of As(V) to the structure and ligand exchange with tunnel site SO . The exchange ratio between As(V) sorption and SO
. The exchange ratio between As(V) sorption and SO release, and the SO
release, and the SO coordination environment in schwertmannite indicates that monodentate As(V) coordination occurs in surface sites while bidentate binuclear As(V) coordination occurs in tunnel sites. Sorption modeling that considers the different types of reactive sites successfully described the observed As(V) sorption behavior.
coordination environment in schwertmannite indicates that monodentate As(V) coordination occurs in surface sites while bidentate binuclear As(V) coordination occurs in tunnel sites. Sorption modeling that considers the different types of reactive sites successfully described the observed As(V) sorption behavior.
 F-FDG Positron Emission Tomography using voxel phantoms
F-FDG Positron Emission Tomography using voxel phantomsTsuda, Keisuke; Kinase, Sakae; Fukushi, Masahiro*; Saito, Kimiaki
no journal, ,
no abstracts in English
Tsuda, Keisuke; Fukushi, Masahiro*; Myojoyama, Atsushi*; Kitamura, Hideaki*; Inoue, Kazumasa*; Nakaya, Giichiro*; Kimura, Junichi*; Sawaguchi, Masato*; Kinase, Sakae; Saito, Kimiaki
no journal, ,
Positron emission tomography (PET) is very effective in the diagnosis and management of patients with various types of cancers. PET scanning with the tracer FDG is widely used in clinical PET. However, the effective dose constant of the positron emitter is about eight times of nuclide  Tc used in a nuclear medicine diagnosis widely. Severe radiation protection is necessary for development of the examination with a positron emitter. Radiation protection in the PET institution therefore and safe security are problems. Hence, lead glass has attracted considerable attention as the radiation shielding materials of the PET institution. In the present study, we received a request of the radiation shielding ability evaluation of two kinds of lead glass made in Pilkington plc. The aim of the present work is the radiation shielding ability evaluation for positron emitter such as
Tc used in a nuclear medicine diagnosis widely. Severe radiation protection is necessary for development of the examination with a positron emitter. Radiation protection in the PET institution therefore and safe security are problems. Hence, lead glass has attracted considerable attention as the radiation shielding materials of the PET institution. In the present study, we received a request of the radiation shielding ability evaluation of two kinds of lead glass made in Pilkington plc. The aim of the present work is the radiation shielding ability evaluation for positron emitter such as  F(511 keV) of the lead glass. The shielding ability evaluation has been studied in the experimental and the Monte Carlo simulation evaluation. Consequently, effective dose transmission factor with experimental and the Monte Carlo simulation value was calculated. There were enough protection effects to evaluated lead glass. Furthermore, radiation shielding ability evaluation for
F(511 keV) of the lead glass. The shielding ability evaluation has been studied in the experimental and the Monte Carlo simulation evaluation. Consequently, effective dose transmission factor with experimental and the Monte Carlo simulation value was calculated. There were enough protection effects to evaluated lead glass. Furthermore, radiation shielding ability evaluation for  Cs(662 keV),
Cs(662 keV),  Co(1.17, 1.33 MeV) was performed, and it was shown that there was a similar protection effect.
Co(1.17, 1.33 MeV) was performed, and it was shown that there was a similar protection effect.
Mizuno, Takashi; Takeuchi, Shinji; Abdelgawad, A.*; Fukushi, Keisuke*; Watanabe, Kunio*
no journal, ,
no abstracts in English
Maeda, Koshi*; Hasegawa, Yusuke*; Fukushi, Keisuke*; Yamamoto, Yuhei; Aosai, Daisuke; Mizuno, Takashi
no journal, ,
no abstracts in English
Hasegawa, Yusuke*; Yamamoto, Yuhei; Aosai, Daisuke; Mizuno, Takashi; Maeda, Koshi*; Fukushi, Keisuke*
no journal, ,
no abstracts in English
Maeda, Koshi*; Hasegawa, Yusuke*; Fukushi, Keisuke*; Yamamoto, Yuhei; Aosai, Daisuke; Mizuno, Takashi
no journal, ,
no abstracts in English
Yamamoto, Yuhei; Aosai, Daisuke; Mizuno, Takashi; Fukushi, Keisuke*; Hasegawa, Yusuke*; Maeda, Koshi*
no journal, ,
no abstracts in English
Hasegawa, Yusuke*; Fukushi, Keisuke*; Maeda, Koshi*; Yamamoto, Yuhei; Aosai, Daisuke; Mizuno, Takashi
no journal, ,
Maeda, Koshi*; Fukushi, Keisuke*; Hasegawa, Yusuke*; Yamamoto, Yuhei; Aosai, Daisuke; Mizuno, Takashi
no journal, ,
Maeda, Koshi*; Fukushi, Keisuke*; Hasegawa, Yusuke*; Yamamoto, Yuhei; Aosai, Daisuke; Mizuno, Takashi
no journal, ,
no abstracts in English
Hasegawa, Yusuke*; Fukushi, Keisuke*; Maeda, Koshi*; Yamamoto, Yuhei; Aosai, Daisuke; Mizuno, Takashi
no journal, ,
no abstracts in English
Unami, Kensuke*; Fukushi, Keisuke*; Takahashi, Yoshio*; Itaya, Tetsumaru*; Niwa, Masakazu
no journal, ,
K-Ar dating is generally not applicable to smectite, which is potassium-free clay mineral. However, based on multidisciplinary analyses such as XRD, TEM, exchangeable cation analysis, and XAFS, fixed potassium can exists in clay minerals in which only smectite is identified by XRD analysis.
Unami, Kensuke*; Fukushi, Keisuke*; Takahashi, Yoshio*; Niwa, Masakazu
no journal, ,
Based on our analysis of XAFS, TEM, etc., we found that our analyzed clay minerals include potassium-fixed structure like illite, even if only smectite is identified in them according to XRD analysis.