Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Shoji, Eita*; Isogai, Shosei*; Suzuki, Rikuto*; Kubo, Masaki*; Tsukada, Takao*; Kai, Tetsuya; Shinohara, Takenao; Matsumoto, Yoshihiro*; Fukuyama, Hiroyuki*
Scripta Materialia, 175, p.29 - 32, 2020/01
Times Cited Count:19 Percentile:76.75(Nanoscience & Nanotechnology) on Cu surfaces
on Cu surfacesMoritani, Kosuke*; Tsuda, Muneyuki*; Teraoka, Yuden; Okada, Michio*; Yoshigoe, Akitaka; Fukuyama, Tetsuya*; Kasai, Toshio*; Kasai, Hideaki*
Journal of Physical Chemistry C, 111(27), p.9961 - 9967, 2007/07
Times Cited Count:15 Percentile:45.77(Chemistry, Physical)We report an X-ray photoemission study of the dissociative adsorption of O at Cu(111), (001), and (110) surfaces with an O
at Cu(111), (001), and (110) surfaces with an O molecular beam generated with a variable temperature nozzle. The O-uptake curves, which are produced from precisely measured O-1s peaks, indicate that the dissociative absorption is enhanced as the nozzle temperature is increased up to 1000 K for the normal incidence of O
molecular beam generated with a variable temperature nozzle. The O-uptake curves, which are produced from precisely measured O-1s peaks, indicate that the dissociative absorption is enhanced as the nozzle temperature is increased up to 1000 K for the normal incidence of O at a kinetic energy of 0.5 eV. However, further increasing the nozzle temperature to 1400 K reduces the probability of dissociativeadsorption. These results suggest that vibrational excitations of incident O
at a kinetic energy of 0.5 eV. However, further increasing the nozzle temperature to 1400 K reduces the probability of dissociativeadsorption. These results suggest that vibrational excitations of incident O assist dissociative adsorption while rotational excitations hinder it.
assist dissociative adsorption while rotational excitations hinder it.
 Au surfaces with a hyperthermal O
Au surfaces with a hyperthermal O molecular beam
molecular beamOkada, Michio*; Moritani, Kosuke; Fukuyama, Tetsuya*; Mizutani, Hironori*; Yoshigoe, Akitaka; Teraoka, Yuden; Kasai, Toshio*
Surface Science, 600(18), p.4228 - 4232, 2006/09
Times Cited Count:20 Percentile:64.35(Chemistry, Physical)Dissociative adsorption of hyperthermal O molecules on Cu
molecules on Cu Au(100) was investigated by X-ray photoemission spectroscopy in conjunction with a synchrotron light source. Comparing the O-uptake curve on Cu
Au(100) was investigated by X-ray photoemission spectroscopy in conjunction with a synchrotron light source. Comparing the O-uptake curve on Cu Au with that on Cu, it was found that the dissociative adsorption of O
Au with that on Cu, it was found that the dissociative adsorption of O is more activated (less reactive) due to Au alloying. The low-energy electron-diffraction (LEED) pattern of c(2
is more activated (less reactive) due to Au alloying. The low-energy electron-diffraction (LEED) pattern of c(2 2) for the clean surface turned into the (1
2) for the clean surface turned into the (1 1) LEED pattern during the oxidation by hyperthermal O
1) LEED pattern during the oxidation by hyperthermal O molecular beam, suggesting that the O adsorption induces the Cu-atom segregation on the surface.
molecular beam, suggesting that the O adsorption induces the Cu-atom segregation on the surface.
Moritani, Kosuke; Okada, Michio*; Fukuyama, Tetsuya*; Teraoka, Yuden; Yoshigoe, Akitaka; Kasai, Toshio*
European Physical Journal D, 38(1), p.111 - 115, 2006/04
Times Cited Count:14 Percentile:54.30(Optics)We report a study on the oxidation process induced by a hyperthermal oxygen molecular beam (HOMB) on Cu(110) using X-ray photoemission spectroscopy in conjunction with a synchrotron radiation source. The oxidation process induced by energetic O beams on Cu(110), depending on the azimuthal angle of incidence, suggests that the -Cu-O- added row structure has a role in inhibiting adsorption as a steric obstacle for incident O
beams on Cu(110), depending on the azimuthal angle of incidence, suggests that the -Cu-O- added row structure has a role in inhibiting adsorption as a steric obstacle for incident O molecules.
molecules.
 molecular beams
molecular beamsTeraoka, Yuden; Yoshigoe, Akitaka; Moritani, Kosuke; Takakuwa, Yuji*; Ogawa, Shuichi*; Ishizuka, Shinji*; Okada, Michio*; Fukuyama, Tetsuya*; Kasai, Toshio*
Hoshako, 18(5), p.298 - 309, 2005/09
Representative research results in surface reaction dynamics, performed at a surface chemistry experimental station installed in the JAEA soft X-ray beamline in the SPring-8, were reviewed. As a research result in JAEA, SiO desorption mechanisms in the Si(001) oxidation at high temperature were introduced. As a collaboration with Osaka University on oxidation reaction dynamics of Cu, collision-induced atom absorption process was introduced. As a collaboration with Tohoku University on Ti(0001) oxidation reaction dynamics, it was introduced that two peaks, found in an incident energy dependence of initial sticking probability, were corresponding to potential energy barriers of dissociative adsorption of O molecules.
molecules.
Watanabe, Daisuke*; Che, D.-C.*; Fukuyama, Tetsuya*; Hashinokuchi, Michihiro*; Teraoka, Yuden; Kasai, Toshio*
Review of Scientific Instruments, 76(5), p.055108_1 - 055108_5, 2005/05
Times Cited Count:6 Percentile:33.37(Instruments & Instrumentation)A molecular beam technique for generating an intense pulsed hyperthermal molecular beam (HTMB) was developed. The beam source consists of a pulse valve, a cooling-water bottle that protectes the pulse valve from heat transfer of a high temperature nozzle, and the nozzle with a heater. The pulsed HTMB of HCl was practically generated and characterized by means of (2+1) resonance-enhanced multiphoton ionization and ion time-of-flight techniques.
 Pd(111) surface
Pd(111) surfaceTsuda, Yasutaka*; Makino, Takamasa*; Tsukada, Chie; Yoshigoe, Akitaka; Fukuyama, Tetsuya*; Okada, Michio*
no journal, ,
It is well-known that oxidation is an important chemical reactions in corrosion processes. Cu Pd has an attracting material due to its catalytic property and its oxidation is the important subject. In this presentation, initial oxidation processes at the Cu
Pd has an attracting material due to its catalytic property and its oxidation is the important subject. In this presentation, initial oxidation processes at the Cu Pd(111) surface was studied by using molecular beams. In situ photoemission measurements were conducted using surface chemistry apparatus at BL23SU at SPring-8. Single crystal Cu
Pd(111) surface was studied by using molecular beams. In situ photoemission measurements were conducted using surface chemistry apparatus at BL23SU at SPring-8. Single crystal Cu Pd was used after cleaning by means of Ar ion sputtering and annealing. It was found that molecular beams facilitate oxidation compared to that for backfilling condition. Furthermore, diffusion of surface atoms seems to be driven with increasing surface temperature.
Pd was used after cleaning by means of Ar ion sputtering and annealing. It was found that molecular beams facilitate oxidation compared to that for backfilling condition. Furthermore, diffusion of surface atoms seems to be driven with increasing surface temperature.
 Pt(111) surface with hyperthermal oxygen molecule
Pt(111) surface with hyperthermal oxygen moleculeTsuda, Yasutaka*; Makino, Takamasa*; Tsukada, Chie; Yoshigoe, Akitaka; Fukuyama, Tetsuya*; Okada, Michio*
no journal, ,
Oxidation is a main reaction in corrosion processes and its understabding is important to develop anticorrosion materials. In this study, initial oxidation at Cu Pt(111) surface was investigated by using surface analysis apparatus at BL23SU of SPring-8. The surface oxidized by 2.3 eV molecular beams was analzyed by synchrotron radiation photoelectron spectroscopy. We confirmed that Cu L
Pt(111) surface was investigated by using surface analysis apparatus at BL23SU of SPring-8. The surface oxidized by 2.3 eV molecular beams was analzyed by synchrotron radiation photoelectron spectroscopy. We confirmed that Cu L M
M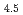 M
M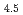 AES spectrum and Cu-2p XPS spectrum show the only growth of Cu oxide whereas Pt oxide was not formed from the results of Pt-4f XPS. It was also found that the reactivity of Cu
AES spectrum and Cu-2p XPS spectrum show the only growth of Cu oxide whereas Pt oxide was not formed from the results of Pt-4f XPS. It was also found that the reactivity of Cu Pd(111) surface is lower than that of Cu
Pd(111) surface is lower than that of Cu Au(111) surface.
Au(111) surface.
Tsuda, Yasutaka*; Makino, Takamasa*; Yoshida, Hikaru; Yoshigoe, Akitaka; Fukuyama, Tetsuya*; Okada, Michio*
no journal, ,
Initial stage of corrosion is one of the central topics in material science. In the present study, we report the results of oxidation on Cu Pd(111) and Cu
Pd(111) and Cu Pt(111) with a hyperthermal O
Pt(111) with a hyperthermal O molecular beam (HOMB) with variable incident energies, using X-ray photoemission spectroscopy in conjunction with synchrotron radiation. All experiments were performed using the Surface Reaction Analysis Apparatus constructed in BL23SU at SPring-8. The Cu
molecular beam (HOMB) with variable incident energies, using X-ray photoemission spectroscopy in conjunction with synchrotron radiation. All experiments were performed using the Surface Reaction Analysis Apparatus constructed in BL23SU at SPring-8. The Cu Pd(111) and Cu
Pd(111) and Cu Pt(111) samples were cleaned by Ar+ sputtering and annealing. The kinetic energy of the HOMB was set to be 2.3 eV. From the uptake curves, it was found that the oxidation of Cu
Pt(111) samples were cleaned by Ar+ sputtering and annealing. The kinetic energy of the HOMB was set to be 2.3 eV. From the uptake curves, it was found that the oxidation of Cu Pt is less effective than that on Cu
Pt is less effective than that on Cu Au and Cu
Au and Cu Pd. This suppression of the surface reactivity may be caused by the Pt-rich subsurface layer.
Pd. This suppression of the surface reactivity may be caused by the Pt-rich subsurface layer.
 Pt(111)
Pt(111)Tsuda, Yasutaka*; Makino, Takamasa*; Yoshida, Hikaru; Yoshigoe, Akitaka; Fukuyama, Tetsuya*; Okada, Michio*
no journal, ,
The oxidation with oxygen molecules is important in a metal corrosion process. In order to develop high noncorrosive materials, the understanding of oxidation processes is significant. In this study, the influence on oxidation reaction due to alloy ingredient difference was investigated by the comparison between Cu Au(111) and Cu
Au(111) and Cu Pt(111) surfaces. It was found that the Cu
Pt(111) surfaces. It was found that the Cu Pt(111) surface has lower reactivity than the Cu
Pt(111) surface has lower reactivity than the Cu Au(111) surface.
Au(111) surface.
 on Cu surfaces
on Cu surfacesMoritani, Kosuke*; Tsuda, Muneyuki*; Teraoka, Yuden; Okada, Michio*; Yoshigoe, Akitaka; Fukuyama, Tetsuya*; Kasai, Toshio*; Kasai, Hideaki*
no journal, ,
Oxygen molecules adsorbe dissociatively at Cu(111), Cu(110), and Cu(001) surfaces. The oxygen uptake curves were investigated by using supersonic molecular beam techniques and photoemission spectroscopy with synchrotron radiation. Adsorption probability increased with increasing translational kinetic energy of oxygen molecules. Vibrational and rotational levels of oxygen molecules were excited by elevation of nozzle temperature up to 1400 K keeping the incident energy of 0.5 eV. Although the adsorption probability increased with increasing nozzle temperature by 1000 K, it decreased inversely around 1400 K. These phenomena were interpreted as adsorption probability increase by vibrational excitation and decrease by rotational excitation.
Okada, Michio*; Hashinokuchi, Michihiro*; Fukuyama, Tetsuya*; Teraoka, Yuden; Kasai, Toshio*
no journal, ,
Oriented supersonic molecular beams of NO and CH Cl molecules, which have a parmanent dipole molent, are generated by non-uniform electric fields. The oriented NO and CH
Cl molecules, which have a parmanent dipole molent, are generated by non-uniform electric fields. The oriented NO and CH Cl beams dissociate and adsorbed at Si(111) and Si(001) surfaces, respectively. Molecular orientation effects for the surface reactions were studied. Sticking probabilities were evaluated in the reactions of oriented CH
Cl beams dissociate and adsorbed at Si(111) and Si(001) surfaces, respectively. Molecular orientation effects for the surface reactions were studied. Sticking probabilities were evaluated in the reactions of oriented CH Cl molecules with the Si(001) surface by the King and Wells method. High reactivity was obtained in the Cl-end collisions. And, elements on the Si(111) surface were analysed by X-ray photoemission spectroscopy in the NO reactions at the Si(111) surface. High reactivity was clarified in the N-end collisions.
Cl molecules with the Si(001) surface by the King and Wells method. High reactivity was obtained in the Cl-end collisions. And, elements on the Si(111) surface were analysed by X-ray photoemission spectroscopy in the NO reactions at the Si(111) surface. High reactivity was clarified in the N-end collisions.