Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ikeda, Atsushi; Tsushima, Satoru*; Takao, Koichiro*; Rossberg, A.*; Funke, H.*; Scheinost, A. C.*; Bernhard, G.*; Yaita, Tsuyoshi; Hennig, C.*
Inorganic Chemistry, 48(24), p.11779 - 11787, 2009/12
Times Cited Count:34 Percentile:79.66(Chemistry, Inorganic & Nuclear)The electrochemical behavior and complex structure of Np carbonato complexes have been investigated in aqueous Na CO
CO and Na
and Na CO
CO /NaOH solutions at different oxidation states by using cyclic voltammetry, X-ray absorption spectroscopy, and density functional theory calculations. The end-member complexes of penta- and hexavalent Np in 1.5 M Na
/NaOH solutions at different oxidation states by using cyclic voltammetry, X-ray absorption spectroscopy, and density functional theory calculations. The end-member complexes of penta- and hexavalent Np in 1.5 M Na CO
CO with pH = 11.7 have been determined as a transdioxo neptunyl tricarbonato complex, [NpO
with pH = 11.7 have been determined as a transdioxo neptunyl tricarbonato complex, [NpO (CO
(CO )
) ]
]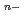 (
( = 5 for Np
= 5 for Np , and 4 for Np
, and 4 for Np ). In contrast, the electrochemical oxidation of Np
). In contrast, the electrochemical oxidation of Np in a highly basic carbonate solution of 2.0M Na
in a highly basic carbonate solution of 2.0M Na CO
CO /1.0M NaOH (pH
/1.0M NaOH (pH  13) yielded a stable heptavalent Np complex of [Np
13) yielded a stable heptavalent Np complex of [Np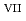 O
O (OH)
(OH) ]
] , indicating that the oxidation reaction from Np
, indicating that the oxidation reaction from Np to Np
to Np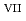 in the carbonate solution involves a drastic structural rearrangement from the transdioxo configuration to a square-planar-tetraoxo configuration, as well as exchanging the coordinating anions from carbonate ions (CO
in the carbonate solution involves a drastic structural rearrangement from the transdioxo configuration to a square-planar-tetraoxo configuration, as well as exchanging the coordinating anions from carbonate ions (CO
 ) to hydroxide ions (OH
) to hydroxide ions (OH ).
).
Ikeda, Atsushi; Hennig, C.*; Rossberg, A.*; Funke, H.*; Scheinost, A. C.*; Bernhard, G.*; Yaita, Tsuyoshi
ESRF Highlights 2008, p.99 - 100, 2009/02
Structural arrangement of Np species has been investigated in various aqueous solutions by synchrotron-based X-ray absorption fine structure (XAFS) spectroscopy. The obtained results revealed that Np(IV) dominantly forms a spherically coordinated decahydrate complex, [Np(H O)
O)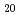 ]
] , while Np(V) and -(VI) form a pentahydrate neptunyl complex, [NpO
, while Np(V) and -(VI) form a pentahydrate neptunyl complex, [NpO (H
(H O)
O) ]
] .
.
Ikeda, Atsushi; Hennig, C.*; Rossberg, A.*; Funke, H.*; Scheinost, A. C.*; Bernhard, G.*; Yaita, Tsuyoshi
Inorganic Chemistry, 47(18), p.8294 - 8305, 2008/09
Times Cited Count:82 Percentile:68.72(Chemistry, Inorganic & Nuclear)Electrochemical and complexation properties of neptunium (Np) are investigated in aqueous perchlorate and nitrate solutions by means of cyclic voltammetry, bulk electrolysis, UV-visible absorption and Np L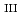 -edge X-ray absorption spectroscopies. The redox reactions of Np(III)/Np(IV) and Np(V)/Np(VI) couples are reversible or quasi-reversible, while the electrochemical reaction between Np(III/IV) and Np(V/VI) is irreversible because they undergo the structural rearrangement from spherical coordinating ions (Np
-edge X-ray absorption spectroscopies. The redox reactions of Np(III)/Np(IV) and Np(V)/Np(VI) couples are reversible or quasi-reversible, while the electrochemical reaction between Np(III/IV) and Np(V/VI) is irreversible because they undergo the structural rearrangement from spherical coordinating ions (Np and Np
and Np ) to transdioxo neptunyl ions (NpO
) to transdioxo neptunyl ions (NpO
 , n = 1 for Np(V) and 2 for Np(VI)). A detailed analysis on extended X-ray absorption fine structure (EXAFS) spectra suggests that Np(IV) forms a decaaquo complex of [Np(H
, n = 1 for Np(V) and 2 for Np(VI)). A detailed analysis on extended X-ray absorption fine structure (EXAFS) spectra suggests that Np(IV) forms a decaaquo complex of [Np(H O)
O) ]
] in 1.0 M HClO
in 1.0 M HClO , while Np(V) and -(VI) exist dominantly as pentaaquo neptunyl complexes, [NpO
, while Np(V) and -(VI) exist dominantly as pentaaquo neptunyl complexes, [NpO (H
(H O)
O) ]
] (n = 1 for Np(V) and 2 for Np(VI)).
(n = 1 for Np(V) and 2 for Np(VI)).