Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
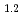 Ni
Ni Mn
Mn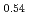 Co
Co O
O in a lithium-ion battery
in a lithium-ion batteryKonishi, Hiroaki*; Hirano, Tatsumi*; Takamatsu, Daiko*; Gunji, Akira*; Feng, X.*; Furutsuki, Sho*; Okumura, Takafumi*; Terada, Shohei*; Tamura, Kazuhisa
Journal of Solid State Chemistry, 262, p.294 - 300, 2018/06
Times Cited Count:9 Percentile:49.17(Chemistry, Inorganic & Nuclear)The potential in each state of charge (SOC) during charging of Li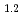 Ni
Ni Mn
Mn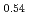 Co
Co O
O is higher than that during discharging. To clarify the effect of chargedischarge operating conditions on the electrochemical reaction, Li
is higher than that during discharging. To clarify the effect of chargedischarge operating conditions on the electrochemical reaction, Li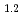 Ni
Ni Mn
Mn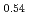 Co
Co O
O was charged and discharged under various charge-discharge operating ranges, and OCP, crystal structure, and oxidation states of the ransition metals were evaluated by electrochemical measurement, XRD, and XAFS. These results indicate that OCP, lattice parameters, and oxidation states of the transition metals of Li
was charged and discharged under various charge-discharge operating ranges, and OCP, crystal structure, and oxidation states of the ransition metals were evaluated by electrochemical measurement, XRD, and XAFS. These results indicate that OCP, lattice parameters, and oxidation states of the transition metals of Li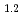 Ni
Ni Mn
Mn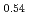 Co
Co O
O in each SOC are not constant. The XRD results indicate that two phases, namely, LiNi
in each SOC are not constant. The XRD results indicate that two phases, namely, LiNi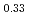 Mn
Mn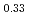 Co
Co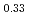 O
O -like and Li
-like and Li MnO
MnO -like, exist in Li
-like, exist in Li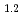 Ni
Ni Mn
Mn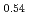 Co
Co O
O .
.
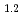 Ni
Ni Mn
Mn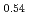 Co
Co O
O used for lithium ion batteries
used for lithium ion batteriesKonishi, Hiroaki*; Hirano, Tatsumi*; Takamatsu, Daiko*; Gunji, Akira*; Feng, X.*; Furutsuki, Sho*; Okumura, Takafumi*; Terada, Shohei*; Tamura, Kazuhisa
Journal of Solid State Chemistry, 258, p.225 - 231, 2018/02
Times Cited Count:8 Percentile:44.83(Chemistry, Inorganic & Nuclear)Li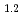 Ni
Ni Mn
Mn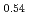 Co
Co O
O is known as one of the cathode electrode material for Li ion batteries and its structure during charge and discharge process was investigated using electrochemical method and X-ray diffraction. It was found that in the charge process the structure changes in the order of Li
is known as one of the cathode electrode material for Li ion batteries and its structure during charge and discharge process was investigated using electrochemical method and X-ray diffraction. It was found that in the charge process the structure changes in the order of Li MnO
MnO , LiNi
, LiNi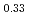 Mn
Mn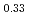 Co
Co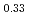 O
O , and Li
, and Li MnO
MnO . On the other hand, in the discharge process, the structure changes in the order of Li
. On the other hand, in the discharge process, the structure changes in the order of Li MnO
MnO and LiNi
and LiNi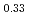 Mn
Mn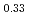 Co
Co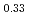 O
O .
.