Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Moritani, Kosuke; Okada, Michio*; Sato, Seiichi*; Goto, Seishiro*; Kasai, Toshio*; Yoshigoe, Akitaka; Teraoka, Yuden
Journal of Vacuum Science and Technology A, 22(4), p.1625 - 1630, 2004/08
Times Cited Count:25 Percentile:66.06(Materials Science, Coatings & Films)We studied the oxidation of Cu{111} surface with a hyperthermal O molecular beam (HOMB) using X-ray photoemission spectroscopy (XPS) in conjunction with a synchrotron radiation (SR) source. The efficiency of oxidation with 0.6-eV-HOMB is higher thab that with 2.3-eV-HOMB under
molecular beam (HOMB) using X-ray photoemission spectroscopy (XPS) in conjunction with a synchrotron radiation (SR) source. The efficiency of oxidation with 0.6-eV-HOMB is higher thab that with 2.3-eV-HOMB under 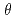
 0.5ML. Ont the other hand, further oxidation occurs rather inefficiency under
0.5ML. Ont the other hand, further oxidation occurs rather inefficiency under 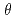
 0.5ML. In this region, efficiency of oxidation with 2.3-eV-HOMB is higher than 0.6-eV-HOMB. We found that such slow oxidation process of Cu can be interpreted in terms of a collision-induced-adsorption mechanism. These results suggest that we can control the oxidation process of Cu by using HOMB.
0.5ML. In this region, efficiency of oxidation with 2.3-eV-HOMB is higher than 0.6-eV-HOMB. We found that such slow oxidation process of Cu can be interpreted in terms of a collision-induced-adsorption mechanism. These results suggest that we can control the oxidation process of Cu by using HOMB.
 molecular beam
molecular beamOkada, Michio*; Moritani, Kosuke; Goto, Seishiro*; Kasai, Toshio*; Yoshigoe, Akitaka; Teraoka, Yuden
Journal of Chemical Physics, 119(14), p.6994 - 6997, 2003/10
Times Cited Count:42 Percentile:78.24(Chemistry, Physical)The oxidation of Cu(001) with hyperthermal O molecular beams was investigated using X-ray photoemission spectroscopy in conjunction with a synchrotron light source. The efficiency of oxidation is higher than that with ambient thermal O
molecular beams was investigated using X-ray photoemission spectroscopy in conjunction with a synchrotron light source. The efficiency of oxidation is higher than that with ambient thermal O . Further oxidation under oxygen coverage larger than 0.5 ML occurs rather inefficiently even for the 2.3 eV beam irradiation. We found such slow oxidation of Cu corresponding to the initial stage of the Cu
. Further oxidation under oxygen coverage larger than 0.5 ML occurs rather inefficiently even for the 2.3 eV beam irradiation. We found such slow oxidation of Cu corresponding to the initial stage of the Cu O formation can be interpreted in terms of a collision-induced-adsorption mechanism. The kinetics of the dissociative adsorption is well described using the first order kinetics in a simple Langmuir-type adsorption model.
O formation can be interpreted in terms of a collision-induced-adsorption mechanism. The kinetics of the dissociative adsorption is well described using the first order kinetics in a simple Langmuir-type adsorption model.
Okada, Michio*; Goto, Seishiro*; Hashinokuchi, Michihiro*; Moritani, Kosuke*; Teraoka, Yuden; Kasai, Toshio*
no journal, ,
We have been developing a new machine of ultra-high-vacuum compatible oriented-molecular beam apparatus. We report results of a study on the incident-energy and the surface-temperature dependence of the steric effects in the dissociative adsorption of CH Cl on an Si(100) surface. The initial sticking probability was measured by the King-Wells method. The data show that the initial sticking probability for the Cl-end collision is larger at an incident energy of 120 meV than that in the CH
Cl on an Si(100) surface. The initial sticking probability was measured by the King-Wells method. The data show that the initial sticking probability for the Cl-end collision is larger at an incident energy of 120 meV than that in the CH -end collision. Furthermore, this steric preference is quite sensitive to the kinetic energy and the rotational state and the surface temperature. This study shows that the non-equilibrium surface trapping plays a key role in the initial step of the decomposition. We also introduce the on-going experiments using a new apparatus for the reaction of oriented NO molecules with Si(111), we found that a N-end collision is more effective for the reaction than O-end collision.
-end collision. Furthermore, this steric preference is quite sensitive to the kinetic energy and the rotational state and the surface temperature. This study shows that the non-equilibrium surface trapping plays a key role in the initial step of the decomposition. We also introduce the on-going experiments using a new apparatus for the reaction of oriented NO molecules with Si(111), we found that a N-end collision is more effective for the reaction than O-end collision.