Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tanaka, Kazuya; Yamaji, Keiko*; Masuya, Hayato*; Tomita, Jumpei; Ozawa, Mayumi*; Yamasaki, Shinya*; Tokunaga, Kohei; Fukuyama, Kenjin*; Ohara, Yoshiyuki*; Maamoun, I.*; et al.
Chemosphere, 355, p.141837_1 - 141837_11, 2024/05
In this study, biogenic Mn(IV) oxide was applied to remove Ra from mine water collected from a U mill tailings pond in the Ningyo-toge center. Just 7.6 mg of biogenic Mn(IV) oxide removed more than 98% of the  Ra from 3 L of mine water, corresponding to a distribution coefficient of 10
Ra from 3 L of mine water, corresponding to a distribution coefficient of 10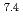 mL/g for Ra at pH 7. The obtained value was convincingly high for practical application of biogenic Mn(IV) oxide in water treatment.
mL/g for Ra at pH 7. The obtained value was convincingly high for practical application of biogenic Mn(IV) oxide in water treatment.
Miyazaki, Kanako*; Takehara, Masato*; Minomo, Kenta*; Horie, Kenji*; Takehara, Mami*; Yamasaki, Shinya*; Saito, Takumi*; Onuki, Toshihiko*; Takano, Masahide; Shiotsu, Hiroyuki; et al.
Journal of Hazardous Materials, 470(15), p.134104_1 - 134104_11, 2024/05
Times Cited Count:1 Percentile:31.14(Engineering, Environmental)Fueda, Kazuki*; Komiya, Tatsuki*; Minomo, Kenta*; Horie, Kenji*; Takehara, Mami*; Yamasaki, Shinya*; Shiotsu, Hiroyuki; Onuki, Toshihiko*; Grambow, B.*; Law, G. T. W.*; et al.
Chemosphere, 328, p.138566_1 - 138566_12, 2023/07
Times Cited Count:5 Percentile:50.39(Environmental Sciences) C control rods in Fukushima Daiichi nuclear reactors during meltdown; B-Li isotopic signatures in cesium-rich microparticles
C control rods in Fukushima Daiichi nuclear reactors during meltdown; B-Li isotopic signatures in cesium-rich microparticlesFueda, Kazuki*; Takami, Ryu*; Minomo, Kenta*; Morooka, Kazuya*; Horie, Kenji*; Takehara, Mami*; Yamasaki, Shinya*; Saito, Takumi*; Shiotsu, Hiroyuki; Onuki, Toshihiko*; et al.
Journal of Hazardous Materials, 428, p.128214_1 - 128214_10, 2022/04
Times Cited Count:12 Percentile:66.41(Engineering, Environmental)Grambow, B.; Nitta, Ayako; Shibata, Atsuhiro; Koma, Yoshikazu; Utsunomiya, Satoshi*; Takami, Ryu*; Fueda, Kazuki*; Onuki, Toshihiko*; Jegou, C.*; Laffolley, H.*; et al.
Journal of Nuclear Science and Technology, 59(1), p.1 - 24, 2022/01
Times Cited Count:32 Percentile:76.55(Nuclear Science & Technology)Guido-Garcia, F.; Sakamoto, Fuminori; David, K.*; Kozai, Naofumi; Grambow, B.
Chemosphere, 279, p.130511_1 - 130511_10, 2021/09
Times Cited Count:1 Percentile:3.45(Environmental Sciences)Cesium (Cs) accumulation by Shiitake was investigated to contribute to the elucidation of radiocesium-cycling mechanisms in forest environments. The results demonstrate that Shiitake non-specifically accumulates Cs while accumulating the essential element K and provide evidence that no selective Cs accumulation (or binding) sites exist within the Shiitake fruit body. Furthermore, the present results show that most accumulated Cs quickly leaches out from the dead fruit body with exposure to water. The leached Cs was largely adsorbable on clay minerals, suggesting that the Shiitake fruit body likely contains Cs in the cation form.
Kimura, Tatsuki*; Fukutani, Satoshi*; Ikegami, Maiko*; Sakamoto, Fuminori; Kozai, Naofumi; Grambow, B.*; Yoneda, Minoru*
Chemosphere, 276, p.130121_1 - 130121_7, 2021/08
Times Cited Count:2 Percentile:7.33(Environmental Sciences)The adsorption of cesium (Cs) on biotite and dissolution of Cs from Cs-bearing biotite using a siderophore were investigated aiming to contribute to the elucidation of radiocesium migration mechanisms in the soil environment. Cs was adsorbed on a hardly weathered biotite powder sample. A siderophore was extracted and purified from the bacterial culture medium, and the purified siderophore was used in five consecutive dissolution experiments of the biotite samples. The major components of the biotite (Al, Fe, and Mg) were dissolved almost stoichiometrically, strongly suggesting that the siderophore selectively dissolves the broken edges of the biotite. The Cs adsorbed on the broken edges was dissolved rapidly as the siderophore dissolved the broken edges, and then, the Cs adsorbed on the outer planar surface of the biotite particles was slowly dissolved.

 on Ni-Zn layered hydroxide salt and its application to removal of ReO
on Ni-Zn layered hydroxide salt and its application to removal of ReO
 as a surrogate of TcO
as a surrogate of TcO

Tanaka, Kazuya; Kozai, Naofumi; Yamasaki, Shinya*; Onuki, Toshihiko; Kaplan, D. I.*; Grambow, B.
Applied Clay Science, 182, p.105282_1 - 105282_8, 2019/12
Times Cited Count:24 Percentile:76.02(Chemistry, Physical)In this study, Ni-Zn layered hydroxide salt (LHS) was used for adsorption experiments of ReO
 , as a surrogate of TcO
, as a surrogate of TcO
 , in aqueous solutions with various initial Re and sodium salt concentrations. The maximum adsorption amount of Re was estimated at 127.7 mg/g (6.86
, in aqueous solutions with various initial Re and sodium salt concentrations. The maximum adsorption amount of Re was estimated at 127.7 mg/g (6.86  10
10 eq/g) by fitting adsorption isotherm of ReO
eq/g) by fitting adsorption isotherm of ReO
 to Langmuir plot. The adsorption of ReO
to Langmuir plot. The adsorption of ReO
 at neutral pH was a reversible process by anion exchange, and decreased with increasing Cl
at neutral pH was a reversible process by anion exchange, and decreased with increasing Cl , NO
, NO
 and SO
and SO
 in solution. EXAFS analysis indicated that ReO
in solution. EXAFS analysis indicated that ReO
 was adsorbed as an outer-sphere complex on Ni-Zn LHS. The Ni-Zn LHS is a more robust adsorbent for ReO
was adsorbed as an outer-sphere complex on Ni-Zn LHS. The Ni-Zn LHS is a more robust adsorbent for ReO
 than the Mg-Al LDH in terms of solution pH and tolerance to competing anions, and may be an effective alternative to the traditional and more limited method of removing aqueous TcO
than the Mg-Al LDH in terms of solution pH and tolerance to competing anions, and may be an effective alternative to the traditional and more limited method of removing aqueous TcO
 by reductive precipitation.
by reductive precipitation.
Tanaka, Kazuya; Kozai, Naofumi; Onuki, Toshihiko; Grambow, B.
Journal of Porous Materials, 26(2), p.505 - 511, 2019/04
Times Cited Count:12 Percentile:40.88(Chemistry, Applied)In this study, we utilized X-ray absorption fine structure spectroscopy to clarify the coordination structure of Re in Mg-Al LDH as a surrogate of Tc. Adsorption experiments of ReO
 on calcined and uncalcined Mg-Al LDHs were conducted in aqueous solutions with different concentrations of NaCl, NaNO
on calcined and uncalcined Mg-Al LDHs were conducted in aqueous solutions with different concentrations of NaCl, NaNO , and Na
, and Na SO
SO . Calcined Mg-Al LDH showed much higher adsorption than uncalcined one. The adsorption of ReO
. Calcined Mg-Al LDH showed much higher adsorption than uncalcined one. The adsorption of ReO
 was reversible, and decreased with increasing concentration of competing anions like Cl
was reversible, and decreased with increasing concentration of competing anions like Cl , NO
, NO
 , or SO
, or SO
 . Analysis of Re L
. Analysis of Re L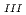 -edge extended X-ray absorption fine structure indicated that ReO
-edge extended X-ray absorption fine structure indicated that ReO
 was adsorbed as an outer-sphere complex on Mg-Al LDH. The observed Re adsorption-desorption behavior, which was sensitive to the presence of competing anions, was consistent with the formation of outer sphere-complex.
was adsorbed as an outer-sphere complex on Mg-Al LDH. The observed Re adsorption-desorption behavior, which was sensitive to the presence of competing anions, was consistent with the formation of outer sphere-complex.
Nagano, Tetsushi; Naganawa, Hirochika; Suzuki, Hideya; Toshimitsu, Masaaki*; Mitamura, Hisayoshi*; Yanase, Nobuyuki*; Grambow, B.
Analytical Sciences, 34(9), p.1099 - 1102, 2018/09
Times Cited Count:14 Percentile:42.99(Chemistry, Analytical)A previously reported emulsion flow (EF) extraction system does not include a device for refining used solvent. Therefore, the processing of large quantities of wastewater by using the EF extractor alone could lead to the accumulation of wastewater components into the solvent and diminished extraction performance. In the present study, we have developed a solvent-washing-type EF system, which is equipped with a unit for washing used solvent to prevent accumulation, and successfully applied it for treating uranium-containing wastewater.
 glycoprotein; Microbial transformation of heavy elements in the aquatic environment
glycoprotein; Microbial transformation of heavy elements in the aquatic environmentKozai, Naofumi; Sakamoto, Fuminori; Tanaka, Kazuya; Onuki, Toshihiko; Sato, Takahiro*; Kamiya, Tomihiro*; Grambow, B.
Chemosphere, 196, p.135 - 144, 2018/04
Times Cited Count:6 Percentile:17.62(Environmental Sciences)Transformation of heavy elements by microbes such as bacteria and fungi has been an intense research subject; however, little is known about that of protozoa. This study investigated interaction of a representative protozoa,  , with heavy elements (Eu(III), Pb(II), U(VI)). Non-destructive elemental analysis by micro-PIXE hardly detected those elements on living cells after sorption experiments but clearly detected on the cells that were killed with a fixative beforehand. Chromatographic analysis of aquatic species of those heavy elements after the sorption experiments revealed a fraction of those elements bound to a glycoprotein dissolved from the cell surface of living
, with heavy elements (Eu(III), Pb(II), U(VI)). Non-destructive elemental analysis by micro-PIXE hardly detected those elements on living cells after sorption experiments but clearly detected on the cells that were killed with a fixative beforehand. Chromatographic analysis of aquatic species of those heavy elements after the sorption experiments revealed a fraction of those elements bound to a glycoprotein dissolved from the cell surface of living  cells to form soluble pseudocolloid. These findings suggest that complexation of heavy elements with the dissolved surface glycoprotein reduced the sorption of those heavy elements on living cells.
cells to form soluble pseudocolloid. These findings suggest that complexation of heavy elements with the dissolved surface glycoprotein reduced the sorption of those heavy elements on living cells.
 Cs uptake by lentinula edodes (shiitake) mushrooms
Cs uptake by lentinula edodes (shiitake) mushroomsGuido-Garcia, F.; Sakamoto, Fuminori; Kozai, Naofumi; Grambow, B.; Devid, K.*
no journal, ,
The uptake of Cs by fungi has been thought to be similar to the uptake of K due to their chemical similarities. In this research we aimed to evaluate the impact of different concentrations of K in Cs uptake during mushrooms growth, as well as determine the spatial distribution of both within fruit bodies. Lentinula edodes (shiitake) was used as a model organism due to their capacity to produce fruit body. Preliminary results have shown that the concentrations of  Cs in the obtained mushroom samples from mushroom bed did not show any clear relationship to the different concentrations of added K. Higher concentrations of
Cs in the obtained mushroom samples from mushroom bed did not show any clear relationship to the different concentrations of added K. Higher concentrations of  Cs were observed in pileus than in stem, consistently. The results of Cs and K mapping did not show a significant spatial variation. This result suggest that Cs and K are distributed evenly, and that there is no specific or selective binding site for cesium.
Cs were observed in pileus than in stem, consistently. The results of Cs and K mapping did not show a significant spatial variation. This result suggest that Cs and K are distributed evenly, and that there is no specific or selective binding site for cesium.
 Cs by shiitake cultivated in mushroom bed
Cs by shiitake cultivated in mushroom bedGuido-Garcia, F.; Kozai, Naofumi; Sakamoto, Fuminori; Grambow, B.
no journal, ,
We aimed to improve the understanding of the mechanisms by which mushrooms can accumulate radiocesium within the fruit body.Cesium-137 is one of the most common fission products of uranium in nuclear reactors and was widely spread during the Fukushima incident in 2011. Nowadays,  Cs is one of the main concerns in terms of contamination due to its half-life (30.1 y) and prevalence in forests. Here, cesium is accumulated by different organisms such as animals, plants and fungi. Specifically for fungi, some species have been identified that can accumulate
Cs is one of the main concerns in terms of contamination due to its half-life (30.1 y) and prevalence in forests. Here, cesium is accumulated by different organisms such as animals, plants and fungi. Specifically for fungi, some species have been identified that can accumulate  Cs efficiently. Mushrooms are of special interest because the ingestion of contaminated species can lead to internal exposure of radioactivity. In this context, understanding the mechanisms by which cesium is accumulated by mushrooms is of particular interest and not yet fully understood. Moreover, the mechanisms by which
Cs efficiently. Mushrooms are of special interest because the ingestion of contaminated species can lead to internal exposure of radioactivity. In this context, understanding the mechanisms by which cesium is accumulated by mushrooms is of particular interest and not yet fully understood. Moreover, the mechanisms by which  Cs is released back into soil after mushrooms die has not been fully investigated either. In this research, we aim to improve the understanding of
Cs is released back into soil after mushrooms die has not been fully investigated either. In this research, we aim to improve the understanding of  Cs uptake by comparing its behavior to another cation (K). For this, shiitake mushrooms were used as a model organism and exposed to
Cs uptake by comparing its behavior to another cation (K). For this, shiitake mushrooms were used as a model organism and exposed to  Cs and K under laboratory conditions. Samples of fruit body were taken at different time points and their radioactivity analyzed by Ge detector to evaluate the uptake at different maturity stages. The results of these experiments will be shown and discussed.
Cs and K under laboratory conditions. Samples of fruit body were taken at different time points and their radioactivity analyzed by Ge detector to evaluate the uptake at different maturity stages. The results of these experiments will be shown and discussed.
 Cs accumulated
Cs accumulated  in Fukushima
in FukushimaSakamoto, Fuminori; Kozai, Naofumi; Guido-Garcia, F.; Grambow, B.
no journal, ,
Much  Cs derived from Fukushima Daiichi Nuclear Power Plant is still staying in the forest area in Fukushima. As a part of elucidation study of the
Cs derived from Fukushima Daiichi Nuclear Power Plant is still staying in the forest area in Fukushima. As a part of elucidation study of the  Cs circulation mechanism in a forest, we measured
Cs circulation mechanism in a forest, we measured  Cs radioactivity of egg mushrooms (
Cs radioactivity of egg mushrooms ( ) which growth wild in Kawamata city in Fukushima at the each growth stages (egg like state, immaturity, maturity, after maturity) and each parts (pileus and stem). As a result, we confirmed they accumulate
) which growth wild in Kawamata city in Fukushima at the each growth stages (egg like state, immaturity, maturity, after maturity) and each parts (pileus and stem). As a result, we confirmed they accumulate  Cs at the stage egg and mature more than the other stages. It is difficult to estimate exactly because the immature sample was slightly one. The radioactivity of
Cs at the stage egg and mature more than the other stages. It is difficult to estimate exactly because the immature sample was slightly one. The radioactivity of  Cs in the samples after maturity decreased. It is suggested that they release again as a fruit bodies die. As the result of comparison of
Cs in the samples after maturity decreased. It is suggested that they release again as a fruit bodies die. As the result of comparison of  Cs radioactivity in the pileus and stem, pileus accumulates
Cs radioactivity in the pileus and stem, pileus accumulates  Cs more than stem.
Cs more than stem.
Sakamoto, Fuminori; Kozai, Naofumi; Guido-Garcia, F.; Kimura, Tatsuki; Grambow, B.
no journal, ,
Soil microorganisms are known to remove a fraction of tightly fixed radiocesium and make it bioavailable. In this study, we attempted to nondestructively recover available radiocesium from soil using microorganisms instead of plants. Microbial activity was tested to form bioavailable radiocesium by the comparing the addition of nutrients and microbicide. Powdered minerals, a water absorbent material, and paper towels were packed in a fine mesh cloth bag (mineral bag) with a size of 50 cm  50 cm. The radiocesium concentration analysis revealed that the surface soil contained approximately 0.3-1.2 Bq/g. The mineral mat was replaced with a new one every two weeks. Two control experiments were established: one with 1 L of nutrient solution to activate microorganisms, and another with sodium hypochlorite aqueous solution to suppress microbial activity. These solutions were added every week. After eight weeks, radiocesium concentrations of the top soil and in the soil core were measured. The accumulative radiocesium concentration in the four mats after eight weeks was within 4.2
50 cm. The radiocesium concentration analysis revealed that the surface soil contained approximately 0.3-1.2 Bq/g. The mineral mat was replaced with a new one every two weeks. Two control experiments were established: one with 1 L of nutrient solution to activate microorganisms, and another with sodium hypochlorite aqueous solution to suppress microbial activity. These solutions were added every week. After eight weeks, radiocesium concentrations of the top soil and in the soil core were measured. The accumulative radiocesium concentration in the four mats after eight weeks was within 4.2 10
10 and 9.0
and 9.0 10
10 Bq, indicating that the mats indeed absorbed radiocesium from soil. The driving force of upward migration of radiocesium is thought to be soil water flow due to continuous water absorption from soil by the mat and evaporation from itself. These results suggest that cesium upward migration flow along with water is a behavior expected to happen in the environment.
Bq, indicating that the mats indeed absorbed radiocesium from soil. The driving force of upward migration of radiocesium is thought to be soil water flow due to continuous water absorption from soil by the mat and evaporation from itself. These results suggest that cesium upward migration flow along with water is a behavior expected to happen in the environment.
Kimura, Tatsuki; Guido-Garcia, F.; Kozai, Naofumi; Zhang, S.*; Yamaji, Keiko*; Yu, Q.*; Grambow, B.
no journal, ,
To understand bacterial ability to dissolve clay minerals, we isolated siderophore-producing bacteria from white clover roots grown in south Osaka, Japan. The ability to produce siderophores was evaluated by CAS plate method. llite, biotite, vermiculite and nontronite were used for testing as clay minerals. Three different bacteria strains were cultivated. Bacteria cells were centrifuged and washed before inoculation tests. Fifty mg dry weight of bacteria were inoculated into 100 mL modified Balland media and 100 mg of each clay mineral added. The suspension was sampled and new media was added. The concentrations of iron, aluminium and silicon were determined by ICP-OES. The presence of organic molecules was determined by SEC-ICP-OES. The peaks of siderophores were determined using the 405 nm absorbance. As a result, siderophore producing bacteria are able to dissolve clay. This suggests that the effect of siderophore on cesium dissolution is an indirectly process.
 Cs using mushroom mycelium and minerals
Cs using mushroom mycelium and mineralsSakamoto, Fuminori; Kozai, Naofumi; Tanaka, Kazuya; Grambow, B.*
no journal, ,
Six years have passed since the Fukushima Daiichi Nuclear Power accident. However, the decontamination of the forest area remains almost untouched. To develop an effective decontamination method for Fukushima forest soils, we first devised the method to estimate radioactivity of Cs-137 accumulated in fungal mycelium through testing Cs-137 accumulation by about 1500 strains of fungi and then prepared a flat decontamination bag composed of nutrient media, the fungal mycelium with a high Cs-137 accumulation efficiency, and a mixture of zeolite, vermiculite and phlogopite. The decontamination bag accumulated Cs-137 from the contaminated soil in Fukushima forest. Without the minerals, the accumulated Cs-137 was released to the environment after the death of the mycelium. By the addition of the minerals, the Cs-137 in the mycelium was stably fixed by the minerals after the death of the mycelium.
Guido-Garcia, F.; Kimura, Tatsuki*; Sakamoto, Fuminori; Kozai, Naofumi; David, K.*; Grambow, B.*; Haruma, Toshikatsu; Yamaji, Keiko*
no journal, ,
Mushrooms (fungi) accumulate radiocesium from soil and believed to control Cs circulation in forest. To elucidate mechanism of fungi-involving radiocesium circulation in forest, we investigated dissolution of mineral by a fungus mycelia (Phlebiopsis gigantean) and local concentration ratios of cesium to potassium, Cs/K, in fruit body of Lentinula edodes (shiitake). The tested fungus has higher ability than a siderophore-producing bacterium to dissolve minerals. Most of the Fe dissolved from minerals were found to be not stable complexes with organic substances. Cs/K values were almost constant in same fruit body specimen of shiitake, revealing that cesium is stored in proportion to potassium at any locations of a shiitake fruit body and there is no specific binding site for cesium in shiitake.
 Cs by mushrooms
Cs by mushroomsGuido-Garcia, F.; Sakamoto, Fuminori; Kozai, Naofumi; Grambow, B.
no journal, ,
 Cs was released from FNPP in 2011 and is one of the main concerns due to its half-life and prevalence in forests. Here, Cs can be taken and accumulated by different organisms, e.g. mushrooms. This can result in mobilization through the environment. Elucidating the mechanisms by which cesium is taken from soil by mushrooms is key to understand its environmental behavior. In soil, Cs can be tightly fixed to clay minerals thus its bioavailability is poor. Organic acids and siderophores produced by mushrooms may remove Cs from mineral surfaces making it bioavailable. To assess this, different strains of fungi were chosen from a ~1000 collection that has been previously tested for Cs accumulation capacity. Organic acids were analyzed by GC-MS and HPLC-ICP-MS from different strains with different accumulating capacities. These were then tested in cesium desorption experiments using key minerals, and compared to a commercial siderophore.
Cs was released from FNPP in 2011 and is one of the main concerns due to its half-life and prevalence in forests. Here, Cs can be taken and accumulated by different organisms, e.g. mushrooms. This can result in mobilization through the environment. Elucidating the mechanisms by which cesium is taken from soil by mushrooms is key to understand its environmental behavior. In soil, Cs can be tightly fixed to clay minerals thus its bioavailability is poor. Organic acids and siderophores produced by mushrooms may remove Cs from mineral surfaces making it bioavailable. To assess this, different strains of fungi were chosen from a ~1000 collection that has been previously tested for Cs accumulation capacity. Organic acids were analyzed by GC-MS and HPLC-ICP-MS from different strains with different accumulating capacities. These were then tested in cesium desorption experiments using key minerals, and compared to a commercial siderophore.
 Cs accumulation and species of mushroom, and proteins expressed under
Cs accumulation and species of mushroom, and proteins expressed under  Cs existence
Cs existenceSakamoto, Fuminori; Guido-Garcia, F.; Kozai, Naofumi; Tanaka, Kazuya; Grambow, B.
no journal, ,
The radiocesium becomes the stress for creatures, but it is known that some plants and fungi collect radiocesium. We continue a study using a mushroom for the purpose of decontamination of the radiocesium by microbes. We tried to confirm species specificity, expressed proteins and so on to elucidate the mechanism of accumulation of radiocesium by mushroom. As a result, we identified 10 species that accumulate  Cs as a high rate and another 10 species that accumulate
Cs as a high rate and another 10 species that accumulate  Cs as a low rate. We checked the specificity of species, but any tendencies, regularity and specificity were not discovered. We only confirmed the tendency that high accumulated species need a long culture period and low accumulated species need not a long culture period. As a result of two-dimensional electrophoretic analysis, we specified 4 kinds of proteins which express under
Cs as a low rate. We checked the specificity of species, but any tendencies, regularity and specificity were not discovered. We only confirmed the tendency that high accumulated species need a long culture period and low accumulated species need not a long culture period. As a result of two-dimensional electrophoretic analysis, we specified 4 kinds of proteins which express under  Cs existence and 5 kinds of proteins which disappeared under
Cs existence and 5 kinds of proteins which disappeared under  Cs absence.
Cs absence.