Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Irisawa, Eriko; Kato, Chiaki; Kamoshida, Michio*; Hakamatsuka, Yasuyuki*; Ueno, Fumiyoshi; Yamamoto, Masahiro
Proceedings of European Corrosion Congress 2017 (EUROCORR 2017) and 20th ICC & Process Safety Congress 2017 (USB Flash Drive), 9 Pages, 2017/09
 ,
, -di(2-ethylhexyl)butanamide-nitric acid systems using turbidity measurements
-di(2-ethylhexyl)butanamide-nitric acid systems using turbidity measurementsTsutsui, Nao; Ban, Yasutoshi; Hakamatsuka, Yasuyuki; Matsumura, Tatsuro
Separation Science and Technology, 51(6), p.961 - 967, 2016/02
Times Cited Count:1 Percentile:4.38(Chemistry, Multidisciplinary)Quantitative evaluation of the two-phase separation between  ,
, -di(2-ethylhexyl)butanamide (DEHBA) and tri-
-di(2-ethylhexyl)butanamide (DEHBA) and tri- -butyl phosphate (TBP) diluted with
-butyl phosphate (TBP) diluted with  -dodecane and uranyl nitrate solution in nitric acid medium was achieved using turbidity measurements. The turbidities of DEHBA were relatively high, particularly at high DEHBA concentrations, while that of TBP rapidly decreased irrespective of nitric acid concentration. A high concentration of DEHBA, nitric acid, and uranium increased the turbidities in the organic phase, which could be ascribed to the increase in viscosity. Distribution ratios of uranium were also measured, and it was indicated that turbidity did not have a critical effect on the distribution ratio when the turbidity was below a certain value.
-dodecane and uranyl nitrate solution in nitric acid medium was achieved using turbidity measurements. The turbidities of DEHBA were relatively high, particularly at high DEHBA concentrations, while that of TBP rapidly decreased irrespective of nitric acid concentration. A high concentration of DEHBA, nitric acid, and uranium increased the turbidities in the organic phase, which could be ascribed to the increase in viscosity. Distribution ratios of uranium were also measured, and it was indicated that turbidity did not have a critical effect on the distribution ratio when the turbidity was below a certain value.
 ,
, -dialkylamides-nitric acid systems
-dialkylamides-nitric acid systemsTsutsui, Nao; Ban, Yasutoshi; Hakamatsuka, Yasuyuki; Urabe, Shunichi; Matsumura, Tatsuro
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.1153 - 1157, 2015/09
 ,
, -Dialkylamides are promising alternative extractants to tri-
-Dialkylamides are promising alternative extractants to tri- -butyl phosphate in the reprocessing of spent nuclear fuels, but the two-phase separation between their organic and aqueous phases has not been evaluated quantitatively.
-butyl phosphate in the reprocessing of spent nuclear fuels, but the two-phase separation between their organic and aqueous phases has not been evaluated quantitatively.  ,
, -Di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) in
-Di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA) in  -dodecane were agitated with uranyl nitrate-containing nitric acid, and their turbidities and their uranium distribution ratios were measured with respect to the time for the quantitative evaluation. Increasing DEHDMPA, uranium, and nitric acid concentrations enhanced turbidities. Although turbidities decreased with respect to the time, uranium distribution ratios slightly changed, indicating the observed turbidities did not affect these uranium distribution ratios significantly. Therefore, DEHDMPA may act as suitable extractant for uranium in nitric acid from two-phase separation viewpoint, and turbidity may be an indicator for extractant performance evaluation.
-dodecane were agitated with uranyl nitrate-containing nitric acid, and their turbidities and their uranium distribution ratios were measured with respect to the time for the quantitative evaluation. Increasing DEHDMPA, uranium, and nitric acid concentrations enhanced turbidities. Although turbidities decreased with respect to the time, uranium distribution ratios slightly changed, indicating the observed turbidities did not affect these uranium distribution ratios significantly. Therefore, DEHDMPA may act as suitable extractant for uranium in nitric acid from two-phase separation viewpoint, and turbidity may be an indicator for extractant performance evaluation.
 nitric acid at elevated temperatures
nitric acid at elevated temperaturesBan, Yasutoshi; Hakamatsuka, Yasuyuki; Tsutsui, Nao; Urabe, Shunichi; Hagiya, Hiromichi; Matsumura, Tatsuro
Radiochimica Acta, 102(9), p.775 - 780, 2014/09
Times Cited Count:5 Percentile:34.55(Chemistry, Inorganic & Nuclear)Optical absorption spectra of Np in 3 mol/dm nitric acid at elevated temperatures were measured using an optical glass cell with a water jacket having a light path of 1 cm, and molar extinction coefficients of Np(VI),
nitric acid at elevated temperatures were measured using an optical glass cell with a water jacket having a light path of 1 cm, and molar extinction coefficients of Np(VI), 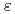
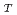 , were obtained at various temperature. The values of
, were obtained at various temperature. The values of 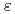
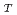 was found to decrease with increasing the temperature, and could be described by the equation
was found to decrease with increasing the temperature, and could be described by the equation 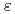
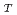 = -0.14
= -0.14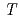 +85.5, where
+85.5, where 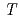 is the temperature. Oxidation of Np(V) to Np(VI) in 3 mol/dm
is the temperature. Oxidation of Np(V) to Np(VI) in 3 mol/dm nitric acid at elevated temperatures was observed using the optical glass cell. Oxidation of Np(V) proceeded as pseudo-first order with respect to Np(V) concentration. The rate equation in the temperature range of 336-362 K was obtained as follows: -d[Np(V)]
nitric acid at elevated temperatures was observed using the optical glass cell. Oxidation of Np(V) proceeded as pseudo-first order with respect to Np(V) concentration. The rate equation in the temperature range of 336-362 K was obtained as follows: -d[Np(V)]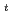 /dt = 2.2
/dt = 2.2 10
10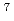 exp[-65
exp[-65 10
10 /(
/(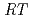 )][Np(V)]
)][Np(V)]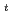 , where
, where 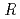 and [Np(V)]
and [Np(V)]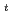 indicate the gas constant and Np(V) concentration at time
indicate the gas constant and Np(V) concentration at time 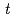 , respectively.
, respectively.
Kato, Chiaki; Ueno, Fumiyoshi; Yamamoto, Masahiro; Hakamatsuka, Yasuyuki; Ban, Yasutoshi; Morita, Yasuji; Uchiyama, Gunzo; Nojima, Yasuo*; Fujine, Sachio*
Zairyo To Kankyo, 60(2), p.69 - 71, 2011/02
It was reported that the metric ions such as Pu and Np contained in reprocessing solutions would accelerate corrosion of stainless steals due to those ions changing higher oxidizer. It's difficult that those ions was used in laboratory test because of radioactive elements. However it's important to understand oxide stats of those ions and electrochemical behavior on stainless steals in order to estimate corrosion rate of materials in reprocessing plats. Furthermore laboratory test with very a little solution volume is demanded for handling radioactive elements. This paper shows that developed a small electrochemical cell with a spectral analysis function and those results of polarization curves on stainless steals and oxide stats of Np(IV)/Np(V) and Pu(IV)/Pu(VI) in boiling nitric acid solutions.
Kato, Chiaki; Ueno, Fumiyoshi; Yamamoto, Masahiro; Hakamatsuka, Yasuyuki; Ban, Yasutoshi; Morita, Yasuji; Uchiyama, Gunzo; Nojima, Yasuo*; Fujine, Sachio*
Fushoku Boshoku Kyokai Dai-57-Kai Zairyo To Kankyo Toronkai Koenshu, p.43 - 46, 2010/10
Reprocessing process solution including Pu and Np may accelerate corrosion of the stainless steel because of oxidizing states in boiling nitric acid. The small electrochemical test cell with a spectral analysis function was developed for the purpose of evaluating corrosion of the stainless steel and oxidizing states of Pu and Np ions in a boiling nitric acid solution. The relationship between corrosion states of the stainless steel and oxidizing states in boiling nitric acid solution can be confirmed.
 ,
, -dialkylamides-nitric acid systems by turbidity measurement
-dialkylamides-nitric acid systems by turbidity measurementTsutsui, Nao; Ban, Yasutoshi; Hakamatsuka, Yasuyuki; Matsumura, Tatsuro
no journal, ,
 ,
, -dialkylamides (monoamides) have been proposed as alternative extractants to tri-
-dialkylamides (monoamides) have been proposed as alternative extractants to tri- -butyl phosphate in the development of spent nuclear fuel reprocessing. Although quantitative evaluation for two-phase separation between nitric acid and monoamides is required for applying monoamides to reprocessing, such evaluation has not been reported. In the present study, the property of two-phase separation between nitric acid and monoamides diluted with dodecane were measured using a turbidimeter. The obtained results showed that the two-phase separation between nitric acid and monoamides was classified into a rapid separation step and a slow separation step, and the time required for the two-phase separation increased with increasing the monoamides concentration. Those results suggested that the turbidity measurement was useful as a method for evaluating a two-phase separation quantitative property.
-butyl phosphate in the development of spent nuclear fuel reprocessing. Although quantitative evaluation for two-phase separation between nitric acid and monoamides is required for applying monoamides to reprocessing, such evaluation has not been reported. In the present study, the property of two-phase separation between nitric acid and monoamides diluted with dodecane were measured using a turbidimeter. The obtained results showed that the two-phase separation between nitric acid and monoamides was classified into a rapid separation step and a slow separation step, and the time required for the two-phase separation increased with increasing the monoamides concentration. Those results suggested that the turbidity measurement was useful as a method for evaluating a two-phase separation quantitative property.
 ,
, -dialkylamides-nitric acid systems by turbidity measurement
-dialkylamides-nitric acid systems by turbidity measurementTsutsui, Nao; Hakamatsuka, Yasuyuki; Ban, Yasutoshi
no journal, ,
 ,
, -dialkylamides (monoamides) have been proposed as alternative extractants to tri-n-butyl phosphate (TBP) in the development of spent nuclear fuel reprocessing. Up to now, however, quantitative estimation of two-phase separation between nitric acid and monoamides has not been carried out. The property of two-phase separation between monoamides (
-dialkylamides (monoamides) have been proposed as alternative extractants to tri-n-butyl phosphate (TBP) in the development of spent nuclear fuel reprocessing. Up to now, however, quantitative estimation of two-phase separation between nitric acid and monoamides has not been carried out. The property of two-phase separation between monoamides ( ,
, -di(2-ethylhexyl)-butanamide (DEHBA) and
-di(2-ethylhexyl)-butanamide (DEHBA) and  ,
, -di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA)) and TBP diluted by dodecane and nitric acid were studied using by a turbidity analyzer. In the case of TBP, two-phase separation proceeded rapidly regardless of the nitric acid concentration. The time require for two-phase separation decreased with increasing the nitric acid concentration in the presence of DEHBA. It took relatively long time for two-phase separation in the DEHDMPA-nitric acid system compared with the other two systems, and the separation time was independent of the nitric acid concentration.
-di(2-ethylhexyl)-2,2-dimethylpropanamide (DEHDMPA)) and TBP diluted by dodecane and nitric acid were studied using by a turbidity analyzer. In the case of TBP, two-phase separation proceeded rapidly regardless of the nitric acid concentration. The time require for two-phase separation decreased with increasing the nitric acid concentration in the presence of DEHBA. It took relatively long time for two-phase separation in the DEHDMPA-nitric acid system compared with the other two systems, and the separation time was independent of the nitric acid concentration.
Ban, Yasutoshi; Morita, Yasuji; Hakamatsuka, Yasuyuki; Kato, Chiaki; Yamamoto, Masahiro; Uchiyama, Gunzo; Nojima, Yasuo*; Fujine, Sachio*
no journal, ,
Polarization curves, open circuit potential, optical absorption spectra were measured in nitric acid solutions containing Np and Pu using SUS310Nb as a working electrode for a study on ageing behavior of reprocessing plants. Appreciable difference was not observed between the polarization curves obtained in presence of Np only and obtained coexistence of Np and Pu.
 ,
, -dialkylamides-nitric acid systems by turbidity measurement, 2; The Correlation between turbidity and the distribution ratio of uranium
-dialkylamides-nitric acid systems by turbidity measurement, 2; The Correlation between turbidity and the distribution ratio of uraniumTsutsui, Nao; Ban, Yasutoshi; Hakamatsuka, Yasuyuki; Urabe, Shunichi; Matsumura, Tatsuro
no journal, ,
The turbidity of the agitated solutions of  ,
, -di(2-ethylhexyl)-butanamide (DEHBA) in dodecane and uranyl nitrate in 3 M HNO
-di(2-ethylhexyl)-butanamide (DEHBA) in dodecane and uranyl nitrate in 3 M HNO was measured for 30 min. The variation of the distribution ratio of uranium after stopping the agitation was also measured, and the correlation between the turbidity and the distribution ratio was studied. The turbidity and the distribution ratio of uranium at 3 min after stopping the agitation was more than 1000 FTU and 1.7, respectively, when the initial uranium concentration was 700 mM. The turbidity at 6 min decreased to 363 FTU, while the distribution rate increased to 2.2. Although the turbidity after 6 min decreased gradually, the distribution ratio barely changed. The correlation between the turbidity and the distribution ratio is discussed.
was measured for 30 min. The variation of the distribution ratio of uranium after stopping the agitation was also measured, and the correlation between the turbidity and the distribution ratio was studied. The turbidity and the distribution ratio of uranium at 3 min after stopping the agitation was more than 1000 FTU and 1.7, respectively, when the initial uranium concentration was 700 mM. The turbidity at 6 min decreased to 363 FTU, while the distribution rate increased to 2.2. Although the turbidity after 6 min decreased gradually, the distribution ratio barely changed. The correlation between the turbidity and the distribution ratio is discussed.
Kim, S.-Y.; Morita, Yasuji; Hakamatsuka, Yasuyuki; Ueno, Fumiyoshi; Yamamoto, Masahiro; Uchiyama, Gunzo; Nojima, Yasuo*; Fujine, Sachio*
no journal, ,
Electrochemical data of Pu and Np at stainless steel electrode and platinum electrode are obtained as fundamental data for the effect of these elements on the material corrosion at the boiling surface. Natural electric potential became constant within 1 hour and the existence of these elements increased the potential.