Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Fujiwara, Hidenori*; Umetsu, Rie*; Kuroda, Fumiaki*; Miyawaki, Jun*; Kashiuchi, Toshiyuki*; Nishimoto, Kohei*; Nagai, Kodai*; Sekiyama, Akira*; Irizawa, Akinori*; Takeda, Yukiharu; et al.
Scientific Reports (Internet), 11(1), p.18654_1 - 18654_9, 2021/09
Times Cited Count:0 Percentile:0(Multidisciplinary Sciences)Cui, Y.-T.*; Harada, Yoshihisa*; Niwa, Hideharu*; Oshima, Masaharu*; Hatanaka, Tatsuya*; Nakamura, Naoki*; Ando, Masaki*; Yoshida, Toshihiko*; Ishii, Kenji*; Matsumura, Daiju
NanotechJapan Bulletin (Internet), 11(4), 6 Pages, 2018/08
no abstracts in English
 VAl probed by soft X-ray spectroscopies
VAl probed by soft X-ray spectroscopiesNagai, Kodai*; Fujiwara, Hidenori*; Aratani, Hidekazu*; Fujioka, Shuhei*; Yomosa, Hiroshi*; Nakatani, Yasuhiro*; Kiss, Takayuki*; Sekiyama, Akira*; Kuroda, Fumiaki*; Fujii, Hitoshi*; et al.
Physical Review B, 97(3), p.035143_1 - 035143_8, 2018/01
Times Cited Count:21 Percentile:70.17(Materials Science, Multidisciplinary)We have studied the electronic structure of ferrimagnetic Mn VAl single crystals by means of soft X-ray absorption spectroscopy (XAS), X-ray absorption magnetic circular dichroism (XMCD), and resonant soft X-ray inelastic scattering (RIXS). We have successfully observed the XMCD signals for all the constituent elements. The Mn L
VAl single crystals by means of soft X-ray absorption spectroscopy (XAS), X-ray absorption magnetic circular dichroism (XMCD), and resonant soft X-ray inelastic scattering (RIXS). We have successfully observed the XMCD signals for all the constituent elements. The Mn L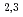 XAS and XMCD spectra are reproduced by spectral simulations based on density-functional theory, indicating the itinerant character of the Mn 3
XAS and XMCD spectra are reproduced by spectral simulations based on density-functional theory, indicating the itinerant character of the Mn 3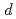 states. On the other hand, the V 3
states. On the other hand, the V 3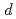 electrons are rather localized since the ionic model can qualitatively explain the V L
electrons are rather localized since the ionic model can qualitatively explain the V L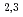 XAS and XMCD spectra. This picture is consistent with local
XAS and XMCD spectra. This picture is consistent with local 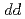 excitations revealed by the V L
excitations revealed by the V L RIXS.
RIXS.
Nakajima, Kenji; Kawakita, Yukinobu; Ito, Shinichi*; Abe, Jun*; Aizawa, Kazuya; Aoki, Hiroyuki; Endo, Hitoshi*; Fujita, Masaki*; Funakoshi, Kenichi*; Gong, W.*; et al.
Quantum Beam Science (Internet), 1(3), p.9_1 - 9_59, 2017/12
The neutron instruments suite, installed at the spallation neutron source of the Materials and Life Science Experimental Facility (MLF) at the Japan Proton Accelerator Research Complex (J-PARC), is reviewed. MLF has 23 neutron beam ports and 21 instruments are in operation for user programs or are under commissioning. A unique and challenging instrumental suite in MLF has been realized via combination of a high-performance neutron source, optimized for neutron scattering, and unique instruments using cutting-edge technologies. All instruments are/will serve in world-leading investigations in a broad range of fields, from fundamental physics to industrial applications. In this review, overviews, characteristic features, and typical applications of the individual instruments are mentioned.
 edge
edgeIshii, Kenji*; Toyama, Takami*; Asano, Shun*; Sato, Kentaro*; Fujita, Masaki*; Wakimoto, Shuichi; Tsutsui, Kenji*; Sota, Shigetoshi*; Miyawaki, Jun*; Niwa, Hideharu*; et al.
Physical Review B, 96(11), p.115148_1 - 115148_8, 2017/09
Times Cited Count:29 Percentile:77.9(Materials Science, Multidisciplinary)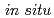 high energy resolution X-ray absorption spectroscopy
high energy resolution X-ray absorption spectroscopyCui, Y.-T.*; Harada, Yoshihisa*; Niwa, Hideharu*; Hatanaka, Tatsuya*; Nakamura, Naoki*; Ando, Masaki*; Yoshida, Toshihiko*; Ishii, Kenji*; Matsumura, Daiju; Oji, Hiroshi*; et al.
Scientific Reports (Internet), 7(1), p.1482_1 - 1482_8, 2017/05
Times Cited Count:19 Percentile:49.58(Multidisciplinary Sciences) and O
and O /H
/H O adsorption revealed by in situ XAFS and hard X-ray photoelectron spectroscopy
O adsorption revealed by in situ XAFS and hard X-ray photoelectron spectroscopyCui, Y.*; Harada, Yoshihisa*; Hatanaka, Tatsuya*; Nakamura, Naoki*; Ando, Masaki*; Yoshida, Toshihiko*; Ikenaga, Eiji*; Ishii, Kenji*; Matsumura, Daiju; Li, R.*; et al.
ECS Transactions, 72(8), p.131 - 136, 2016/10
Times Cited Count:1 Percentile:48.91(Electrochemistry)
Nomura, Takuji*; Harada, Yoshihisa*; Niwa, Hideharu*; Ishii, Kenji*; Ishikado, Motoyuki*; Shamoto, Shinichi; Jarrige, I.*
Physical Review B, 94(3), p.035134_1 - 035134_9, 2016/07
Times Cited Count:11 Percentile:47.12(Materials Science, Multidisciplinary)Low-energy electron excitation spectra were measured on a single crystal of a typical iron-based superconductor PrFeAsO using resonant inelastic X-ray scattering (RIXS) at the Fe-
using resonant inelastic X-ray scattering (RIXS) at the Fe-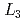 edge.
edge.
 -alkylated 2-pyrrolidone derivatives
-alkylated 2-pyrrolidone derivativesTakao, Koichiro*; Kawata, Yoshihisa*; Nogami, Masanobu*; Harada, Masayuki*; Morita, Yasuji; Nishimura, Kenji*; Ikeda, Yasuhisa*
Journal of Nuclear Science and Technology, 52(2), p.294 - 298, 2015/02
Times Cited Count:2 Percentile:17.57(Nuclear Science & Technology)Yields of precipitated UO (NO
(NO )
) (NRP)
(NRP) (NRP =
(NRP =  -alkylated 2-pyrrolidone) were precisely determined by considering reduction of the solution volume through the precipitation, which can be estimated from difference in acid concentrations of the liquid phases before and after the precipitation. The studied NRPs were
-alkylated 2-pyrrolidone) were precisely determined by considering reduction of the solution volume through the precipitation, which can be estimated from difference in acid concentrations of the liquid phases before and after the precipitation. The studied NRPs were  -
- -butyl (NBP) and
-butyl (NBP) and  -
- -propyl (NProP) derivatives. In both systems, the precipitation yields precisely determined were always higher than those simply calculated from the ratio of uranium concentrations before and after the precipitation. However, the differences between them are in the range of 0.6% - 2.6%. If such a difference is practically negligible, the volume reduction through the precipitation does not have to be taken into account for simplicity of the analytical manipulation.
-propyl (NProP) derivatives. In both systems, the precipitation yields precisely determined were always higher than those simply calculated from the ratio of uranium concentrations before and after the precipitation. However, the differences between them are in the range of 0.6% - 2.6%. If such a difference is practically negligible, the volume reduction through the precipitation does not have to be taken into account for simplicity of the analytical manipulation.
 -type ferromagnetic semiconductor (In,Fe)As
-type ferromagnetic semiconductor (In,Fe)AsKobayashi, Masaki*; Anh, L. D.*; Hai, P. N.*; Takeda, Yukiharu; Sakamoto, Shoya*; Kadono, Toshiharu*; Okane, Tetsuo; Saito, Yuji; Yamagami, Hiroshi; Harada, Yoshihisa*; et al.
Applied Physics Letters, 105(3), p.032403_1 - 032403_4, 2014/07
Times Cited Count:6 Percentile:26.85(Physics, Applied)Kobayashi, Masaki*; Muneta, Iriya*; Takeda, Yukiharu; Harada, Yoshihisa*; Fujimori, Atsushi*; Krempask , J.*; Schmitt, T.*; Oya, Shinobu*; Tanaka, Masaaki*; Oshima, Masaharu*; et al.
, J.*; Schmitt, T.*; Oya, Shinobu*; Tanaka, Masaaki*; Oshima, Masaharu*; et al.
Physical Review B, 89(20), p.205204_1 - 205204_8, 2014/05
Times Cited Count:78 Percentile:92.27(Materials Science, Multidisciplinary) -ray irradiation in HNO
-ray irradiation in HNO media
mediaNogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 296(1), p.423 - 427, 2013/04
Times Cited Count:5 Percentile:38.62(Chemistry, Analytical)Stability of polyvinylpolypyrrolidone (PVPP), a resin with adsorption selectivity to U(VI) in nitric acid media, against  -ray irradiation has been examined using HNO
-ray irradiation has been examined using HNO solutions of various concentrations. As the result, no clear decrease in the capacity was observed for any samples. Or rather, it was found that the capacity increased by approximately 50% for the PVPP slurry irradiated in 6 M HNO
solutions of various concentrations. As the result, no clear decrease in the capacity was observed for any samples. Or rather, it was found that the capacity increased by approximately 50% for the PVPP slurry irradiated in 6 M HNO . The infrared spectroscopic study indicates that PVPP degrades by
. The infrared spectroscopic study indicates that PVPP degrades by  -ray irradiation in HNO
-ray irradiation in HNO from the cleavage of the pyrrolidone ring by the addition of oxygen atom originating from HNO
from the cleavage of the pyrrolidone ring by the addition of oxygen atom originating from HNO , followed by the formation of chain monoamides with multiple coordinative atoms by the continuous addition of oxygen, finally leading to the generation of primary-amine type anion exchange resin. It is also indicated that all generated functional groups possess adsorptivity to U(VI) in 3 M HNO
, followed by the formation of chain monoamides with multiple coordinative atoms by the continuous addition of oxygen, finally leading to the generation of primary-amine type anion exchange resin. It is also indicated that all generated functional groups possess adsorptivity to U(VI) in 3 M HNO .
.
Niwa, Hideharu*; Saito, Makoto*; Kobayashi, Masaki*; Harada, Yoshihisa*; Oshima, Masaharu*; Moriya, Shogo*; Matsubayashi, Katsuyuki*; Nabae, Yuta*; Kuroki, Shigeki*; Ikeda, Takashi; et al.
Journal of Power Sources, 223, p.30 - 35, 2013/02
Times Cited Count:18 Percentile:50.94(Chemistry, Physical)To design non-platinum, inexpensive, but high performance carbon-based cathode catalysts for polymer electrolyte fuel cells, it is important to elucidate the active site for oxygen reduction reaction (ORR). However, it is difficult to directly identify the active site by applying conventional structural or electronic probes to such complex systems. Here, we used C 1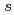 X-ray absorption spectroscopy (XAS) to observe electronic structure of carbon in iron phthalocyanine-based catalysts, and found a signature of edge exposure below the
X-ray absorption spectroscopy (XAS) to observe electronic structure of carbon in iron phthalocyanine-based catalysts, and found a signature of edge exposure below the 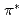 edge, whose intensity is well correlated with the ORR activity. These results demonstrate that C 1
edge, whose intensity is well correlated with the ORR activity. These results demonstrate that C 1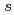 XAS can be used to characterize the ORR activity of carbon-based cathode catalysts in terms of the edge exposure.
XAS can be used to characterize the ORR activity of carbon-based cathode catalysts in terms of the edge exposure.
Kiuchi, Hisao*; Niwa, Hideharu*; Kobayashi, Masaki*; Harada, Yoshihisa*; Oshima, Masaharu*; Chokai, Masayuki*; Nabae, Yuta*; Kuroki, Shigeki*; Kakimoto, Masaaki*; Ikeda, Takashi; et al.
Electrochimica Acta, 82(1), p.291 - 295, 2012/10
Times Cited Count:14 Percentile:34.42(Electrochemistry)We study the characteristics of oxygen adsorption on metal-free carbon-based cathode catalysts derived from nitrogen-containing polyamide (PA) and nitrogen-free phenolic resin (PhRs). Electrochemical analysis and Raman spectroscopy showed higher 2-electron oxygen reduction reaction (ORR) activity and more defect sites in PA than PhRs. The increase in the amount of adsorbed oxygen in PA was also identified by oxygen adsorption isotherms. 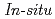 X-ray photoelectron spectroscopy reveals that graphite-like nitrogen contributes to oxygen adsorption and C=O components are dominant in PA. These experimental results indicate that the adsorbed C=O components near the graphite-like nitrogen can be assigned as active sites for 2-electron ORR.
X-ray photoelectron spectroscopy reveals that graphite-like nitrogen contributes to oxygen adsorption and C=O components are dominant in PA. These experimental results indicate that the adsorbed C=O components near the graphite-like nitrogen can be assigned as active sites for 2-electron ORR.
 -ray irradiation
-ray irradiationNogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Science China; Chemistry, 55(9), p.1739 - 1745, 2012/09
Times Cited Count:4 Percentile:19.69(Chemistry, Multidisciplinary)Stability of N-alkylated pyrrolidone derivatives (NRPs) against radiation was examined by irradiation tests with  Co
Co  -ray. We have been developed a novel reprocessing system using NRPs which have precipitation ability to haxa- and tetravalent actinides in nitric acid media. Degradation rates of NRPs are evaluated by irradiation in 3M nitric acid solutions and mechanism of degradation are discussed in the present paper.
-ray. We have been developed a novel reprocessing system using NRPs which have precipitation ability to haxa- and tetravalent actinides in nitric acid media. Degradation rates of NRPs are evaluated by irradiation in 3M nitric acid solutions and mechanism of degradation are discussed in the present paper.
Imazono, Takashi; Moriya, Naoji*; Harada, Yoshihisa*; Sano, Kazuo*; Koike, Masato
AIP Conference Proceedings 1465, p.38 - 42, 2012/07
Times Cited Count:0 Percentile:0.13(Physics, Applied)Kobayashi, Masaki*; Niwa, Hideharu*; Saito, Makoto*; Harada, Yoshihisa*; Oshima, Masaharu*; Ofuchi, Hironori*; Terakura, Kiyoyuki*; Ikeda, Takashi; Koshigoe, Yuka*; Ozaki, Junichi*; et al.
Electrochimica Acta, 74, p.254 - 259, 2012/07
Times Cited Count:52 Percentile:80.98(Electrochemistry)The electronic structure of the residual metal atoms in FePc-based carbon catalysts, prepared by pyrolyzing a mixture of FePc and phenolic resin polymer at 800 C, before and after acid washing have been investigated using XAFS spectroscopy to clarify the role of Fe in the ORR activity. The decomposition analyses for the XAFS spectra reveal that the composition ratio of each Fe component is unaltered by the acid washing, indicating that the residual Fe components were removed by the acid washing irrespective of their chemical states. Because the oxygen reduction potential was approximately unchanged by the acid washing, the residual Fe itself does not seem to contribute directly to the ORR activity. The residual Fe can act as a catalyst to accelerate the growth of the sp
C, before and after acid washing have been investigated using XAFS spectroscopy to clarify the role of Fe in the ORR activity. The decomposition analyses for the XAFS spectra reveal that the composition ratio of each Fe component is unaltered by the acid washing, indicating that the residual Fe components were removed by the acid washing irrespective of their chemical states. Because the oxygen reduction potential was approximately unchanged by the acid washing, the residual Fe itself does not seem to contribute directly to the ORR activity. The residual Fe can act as a catalyst to accelerate the growth of the sp carbon network during pyrolysis. The results imply that light elements are components of the ORR active sites in the FePc-based carbon catalysts.
carbon network during pyrolysis. The results imply that light elements are components of the ORR active sites in the FePc-based carbon catalysts.
Ikeda, Yasuhisa*; Kawasaki, Takeshi*; Harada, Masayuki*; Nogami, Masanobu*; Kawata, Yoshihisa*; Kim, S.-Y.*; Morita, Yasuji; Chikazawa, Takahiro*; Someya, Hiroshi*; Kikuchi, Toshiaki*
Proceedings of International Conference on Toward and Over the Fukushima Daiichi Accident (GLOBAL 2011) (CD-ROM), 5 Pages, 2011/12
An advanced reprocessing system for spent FBR fuels based on two precipitation processes using pyrrolidone derivatives as precipitants has been developed. Experimental results of precipitation behavior of U, Pu and other elements, the heat- and radiation-resistance of precipitants, the thermal decomposition properties of precipitates showed that N-n-butyl-2-pyrrolidone and N-neopentyl-2-pyrrolidone are the appropriate precipitants for the first and second precipitation steps, respectively. From the engineering investigation, We confirmed that the precipitation and the filtration can be done efficiently using the engineering scale equipment and that the fuel pellets are directly prepared by the calcination of the precipitates. On the basis of these results, we evaluated that the proposed system is expected to be one of candidates of the future reprocessing systems for spent FBR fuels.
Kobayashi, Masaki*; Niwa, Hideharu*; Harada, Yoshihisa*; Horiba, Koji*; Oshima, Masaharu*; Ofuchi, Hironori*; Terakura, Kiyoyuki*; Ikeda, Takashi; Koshigoe, Yuka*; Ozaki, Junichi*; et al.
Journal of Power Sources, 196(20), p.8346 - 8351, 2011/10
Times Cited Count:32 Percentile:67.33(Chemistry, Physical)The electronic structure of Co atoms in CoPc-based carbon catalysts, which were prepared by pyrolyzing a mixture of CoPc and phenol resin polymer up to 1000 C, has been investigated using XAFS analysis and HXPES. The Co K XAFS spectra show that most of the Co atoms are in the metallic state and small quantities of oxidized Co components are present in the samples even after acid washing to remove Co atoms. Based on the difference in probing depth between XAFS and HXPES, it was found that after acid washing, the surface region with the aggregated Co clusters is primarily composed of metallic Co. Since the electrochemical properties remain almost unchanged even after the acid washing process, the residual metallic and oxidized Co atoms themselves will hardly contribute to the ORR activity of the CoPc-based carbon cathode catalysts, implying that the active sites of the CoPc-based catalysts primarily consist of light elements such as C and N.
C, has been investigated using XAFS analysis and HXPES. The Co K XAFS spectra show that most of the Co atoms are in the metallic state and small quantities of oxidized Co components are present in the samples even after acid washing to remove Co atoms. Based on the difference in probing depth between XAFS and HXPES, it was found that after acid washing, the surface region with the aggregated Co clusters is primarily composed of metallic Co. Since the electrochemical properties remain almost unchanged even after the acid washing process, the residual metallic and oxidized Co atoms themselves will hardly contribute to the ORR activity of the CoPc-based carbon cathode catalysts, implying that the active sites of the CoPc-based catalysts primarily consist of light elements such as C and N.
Nogami, Masanobu*; Harada, Masayuki*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Progress in Nuclear Energy, 53(7), p.948 - 951, 2011/09
Times Cited Count:3 Percentile:26.02(Nuclear Science & Technology)The precipitation ability of 1,3-dimethyl-2-imidazolidone (DMI) to U(VI) and U(IV) was examined using nitric acid solutions. While DMI precipitated U(VI) from 3 M nitric acid, no precipitate was observed in the solution containing 0.15 M U(IV) and 3 M nitric acid by adding DMI at the ratio of [DMI]/[U(IV)]=5. This indicates that the selectivity of DMI to U(VI) than U(IV). In spite of the excellent selectivity to U(VI), DMI has a disadvantage on the stability in nitric acid, because gradual acid hydrolysis of DMI is inevitable due to the nature of the chemical structure. Experiments on the stability of DMI in  -ray irradiation and heating in nitric acid solutions showed that the stability is strongly affected by the concentration of nitric acid and that DMI may be applicable in nitric acid solutions up to ca. 2 M.
-ray irradiation and heating in nitric acid solutions showed that the stability is strongly affected by the concentration of nitric acid and that DMI may be applicable in nitric acid solutions up to ca. 2 M.