Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nasu, Mitsunori*; Yanai, Hiroshi*; Hirayama, Naoki*; Adachi, Hironori*; Kakizawa, Yu*; Shirase, Yuto*; Nishiyama, Hiromichi*; Kawamoto, Teppei*; Inukai, Junji*; Shinohara, Takenao; et al.
Journal of Power Sources, 530, p.231251_1 - 231251_11, 2022/05
Times Cited Count:32 Percentile:88.79(Chemistry, Physical)Okamura, Hiroyuki; Hirayama, Naoki*
Analytical Sciences, 37(1), p.119 - 130, 2021/01
Times Cited Count:39 Percentile:67.91(Chemistry, Analytical)This review summarizes recent progress in solvent extraction of rare earth elements (REEs) using an ionic liquid (IL) as the extraction solvent. These IL extraction systems are advantageous owing to the affinity of ILs for both charged and neutral hydrophobic species, in contrast to conventional organic solvent extraction systems. Herein, REE extraction studies using ILs are detailed and classified based on the type of extraction system. In IL extraction systems, the extracted complexes are often different from those in organic solvent systems, and the REE extraction and separation efficiencies are often significantly enhanced. Synergistic IL extraction is an effective approach to improving the extractability and separability of REEs. The development of novel task-specific ionic liquids (TSILs) suitable for IL extraction systems is also effective for REE separation.
Okamura, Hiroyuki; Mizuno, Masayoshi*; Hirayama, Naoki*; Shimojo, Kojiro; Naganawa, Hirochika; Imura, Hisanori*
Industrial & Engineering Chemistry Research, 59(1), p.329 - 340, 2020/01
Times Cited Count:15 Percentile:46.87(Engineering, Chemical)The synergistic ionic-liquid extraction and extraction equilibrium of lanthanoid(III) (Ln(III)) ions have been investigated using 2-thenoyltrifluoroacetone (Htta) and trioctylphosphine oxide (TOPO) in the ionic liquid 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C mim][Tf
mim][Tf N]). The selective synergistic effect for heavier Ln(III) ions was found using a combination of Htta and TOPO in [C
N]). The selective synergistic effect for heavier Ln(III) ions was found using a combination of Htta and TOPO in [C mim][Tf
mim][Tf N], leading to enhanced separability among Ln(III) ions. The extracted Ln(III) species and the extraction constants in the Htta-TOPO system were determined by three-dimensional extraction equilibrium analysis. The selective synergism for heavier Ln(III) ions in the Htta-TOPO system was ascribed to the formation of hydrophobic, charged adducts, such as Ln(tta)
N], leading to enhanced separability among Ln(III) ions. The extracted Ln(III) species and the extraction constants in the Htta-TOPO system were determined by three-dimensional extraction equilibrium analysis. The selective synergism for heavier Ln(III) ions in the Htta-TOPO system was ascribed to the formation of hydrophobic, charged adducts, such as Ln(tta) (TOPO)
(TOPO)
 and Ln(tta)(TOPO)
and Ln(tta)(TOPO)
 , in [C
, in [C mim][Tf
mim][Tf N].
N].
Wang, H.*; Otsu, Hideaki*; Chiga, Nobuyuki*; Kawase, Shoichiro*; Takeuchi, Satoshi*; Sumikama, Toshiyuki*; Koyama, Shumpei*; Sakurai, Hiroyoshi*; Watanabe, Yukinobu*; Nakayama, Shinsuke; et al.
Communications Physics (Internet), 2(1), p.78_1 - 78_6, 2019/07
Times Cited Count:10 Percentile:56.67(Physics, Multidisciplinary)Searching for effective pathways for the production of proton- and neutron-rich isotopes through an optimal combination of reaction mechanism and energy is one of the main driving forces behind experimental and theoretical nuclear reaction studies as well as for practical applications in nuclear transmutation of radioactive waste. We report on a study on incomplete fusion induced by deuteron, which contains one proton and one neutron with a weak binding energy and is easily broken up. This reaction study was achieved by measuring directly the cross sections for both proton and deuteron for  Pd at 50 MeV/u via inverse kinematics technique. The results provide direct experimental evidence for the onset of a cross-section enhancement at high energy, indicating the potential of incomplete fusion induced by loosely-bound nuclei for creating proton-rich isotopes and nuclear transmutation of radioactive waste.
Pd at 50 MeV/u via inverse kinematics technique. The results provide direct experimental evidence for the onset of a cross-section enhancement at high energy, indicating the potential of incomplete fusion induced by loosely-bound nuclei for creating proton-rich isotopes and nuclear transmutation of radioactive waste.
Kunieda, Satoshi; Iwamoto, Osamu; Iwamoto, Nobuyuki; Minato, Futoshi; Okamoto, Tsutomu; Sato, Tatsuhiko; Nakashima, Hiroshi; Iwamoto, Yosuke; Iwamoto, Hiroki; Kitatani, Fumito; et al.
JAEA-Conf 2016-004, p.41 - 46, 2016/09
Neutron- and proton-induced cross-section data are required in a wide energy range beyond 20 MeV, for the design of accelerator applications. New evaluations are performed with recent knowledge in the optical and pre-equilibrium model calculations. We also evaluated cross-sections for p+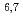 Li and p+
Li and p+ Be which have been highly requested from a medical field. The present high-energy nuclear data library, JENDL-4.0/HE, includes evaluated cross-sections for incident neutrons and protons up to 200 MeV (for about 130 nuclei). We overview substantial features of the library, i.e., (1) systematic evaluation with CCONE code, (2) challenges for evaluations of light nuclei and (3) inheritance of JENDL-4.0 and JENDL/HE-2007. In this talk, we also focus on the results of benchmark calculation for neutronics to show performance of the present library.
Be which have been highly requested from a medical field. The present high-energy nuclear data library, JENDL-4.0/HE, includes evaluated cross-sections for incident neutrons and protons up to 200 MeV (for about 130 nuclei). We overview substantial features of the library, i.e., (1) systematic evaluation with CCONE code, (2) challenges for evaluations of light nuclei and (3) inheritance of JENDL-4.0 and JENDL/HE-2007. In this talk, we also focus on the results of benchmark calculation for neutronics to show performance of the present library.
Okamura, Hiroyuki; Ikeda, Atsushi*; Saito, Takumi*; Aoyagi, Noboru; Naganawa, Hirochika; Hirayama, Naoki*; Umetani, Shigeo*; Imura, Hisanori*; Shimojo, Kojiro
Analytical Chemistry, 84(21), p.9332 - 9339, 2012/11
Times Cited Count:24 Percentile:62.04(Chemistry, Analytical)Okamura, Hiroyuki; Sakae, Hiroki*; Kidani, Keiji*; Hirayama, Naoki*; Aoyagi, Noboru; Saito, Takumi*; Shimojo, Kojiro; Naganawa, Hirochika; Imura, Hisanori*
Polyhedron, 31(1), p.748 - 753, 2012/01
Times Cited Count:29 Percentile:87.38(Chemistry, Inorganic & Nuclear)Okamura, Hiroyuki; Hirayama, Naoki*; Morita, Kotaro*; Shimojo, Kojiro; Naganawa, Hirochika; Imura, Hisanori*
Analytical Sciences, 26(5), p.607 - 611, 2010/05
Times Cited Count:55 Percentile:84.40(Chemistry, Analytical) -diketone fragments on an ionic liquid extraction system
-diketone fragments on an ionic liquid extraction systemShimojo, Kojiro; Okamura, Hiroyuki; Hirayama, Naoki*; Umetani, Shigeo*; Imura, Hisanori*; Naganawa, Hirochika
Dalton Transactions, 2009(25), p.4850 - 4852, 2009/07
A novel extractant  -diketone-substituted diaza-18-crown-6 demonstrated very efficient extraction of Sr
-diketone-substituted diaza-18-crown-6 demonstrated very efficient extraction of Sr due to an intramolecular synergistic effect on the ionic liquid extraction system and recovery of Sr
due to an intramolecular synergistic effect on the ionic liquid extraction system and recovery of Sr from the ionic liquid was successfully achieved under acidic conditions.
from the ionic liquid was successfully achieved under acidic conditions.
; Sakamoto, Yukio; Tanaka, Shunichi; ; Fukahori, Tokio; ; Sasamoto, Nobuo; Tanaka, Susumu; Nakamura, Takashi*; Shin, Kazuo*; et al.
JAERI-Data/Code 94-012, 90 Pages, 1994/09
no abstracts in English
Okamura, Hiroyuki; Sakae, Hiroki*; Kidani, Keiji*; Hirayama, Naoki*; Shimojo, Kojiro; Naganawa, Hirochika; Imura, Hisanori*
no journal, ,
Okamura, Hiroyuki; Shimojo, Kojiro; Ikeda, Atsushi*; Saito, Takumi*; Aoyagi, Noboru; Hirayama, Naoki*; Umetani, Shigeo*; Imura, Hisanori*; Naganawa, Hirochika
no journal, ,
Okamura, Hiroyuki; Shimojo, Kojiro; Ikeda, Atsushi; Hirayama, Naoki*; Umetani, Shigeo*; Imura, Hisanori*; Naganawa, Hirochika
no journal, ,
no abstracts in English
Okamura, Hiroyuki; Shimojo, Kojiro; Hirayama, Naoki*; Imura, Hisanori*; Naganawa, Hirochika
no journal, ,
no abstracts in English
Okamura, Hiroyuki; Shimojo, Kojiro; Hirayama, Naoki*; Umetani, Shigeo*; Imura, Hisanori*; Naganawa, Hirochika
no journal, ,
no abstracts in English
 -diketone fragments
-diketone fragmentsOkamura, Hiroyuki; Shimojo, Kojiro; Hirayama, Naoki*; Umetani, Shigeo*; Imura, Hisanori*; Naganawa, Hirochika
no journal, ,
no abstracts in English
Okamura, Hiroyuki; Shimojo, Kojiro; Hirayama, Naoki*; Imura, Hisanori*; Naganawa, Hirochika
no journal, ,
no abstracts in English
Okamura, Hiroyuki; Mizuno, Masayoshi*; Hirayama, Naoki*; Shimojo, Kojiro; Naganawa, Hirochika; Imura, Hisanori*
no journal, ,
Solvent extraction technique is widely investigated to separate lanthanoids (Ln). The development of a more effective separation system is an important issue in the field of atomic energy. Recently, the synergistic ionic-liquid (IL) extraction of Ln(III) with several  -diketones and trioctylphosphine oxide (TOPO) in 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide was studied, and it was found that the separability of Ln(III) was remarkably enhanced by the selective synergism for heavier rate earth. In this study, the method of extraction equilibrium analysis for Ln(III) in the synergistic ionic-liquid extraction system with 2-thenoyltrifluoroacetone (Htta) and TOPO was investigated. To determine the extraction constants of the respective Ln(III) complexes, a three-dimensional non-linear analysis of log
-diketones and trioctylphosphine oxide (TOPO) in 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide was studied, and it was found that the separability of Ln(III) was remarkably enhanced by the selective synergism for heavier rate earth. In this study, the method of extraction equilibrium analysis for Ln(III) in the synergistic ionic-liquid extraction system with 2-thenoyltrifluoroacetone (Htta) and TOPO was investigated. To determine the extraction constants of the respective Ln(III) complexes, a three-dimensional non-linear analysis of log  -log [tta
-log [tta ]
]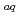 -log [TOPO]
-log [TOPO]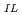 was applied. The formation constants of various ternary complexes formed in IL were calculated, and the order of the formation constants of the Ln(tta)
was applied. The formation constants of various ternary complexes formed in IL were calculated, and the order of the formation constants of the Ln(tta) (TOPO)
(TOPO)
 complexes was found to be Eu(III)
complexes was found to be Eu(III)  Nd(III). Consequently, it was suggested that the formation of hydrophobic charged complexes is a factor for the selective synergism.
Nd(III). Consequently, it was suggested that the formation of hydrophobic charged complexes is a factor for the selective synergism.
Okamura, Hiroyuki; Mizuno, Masayoshi*; Hirayama, Naoki*; Shimojo, Kojiro; Naganawa, Hirochika; Imura, Hisanori*
no journal, ,
We studied the ionic liquid (IL) extraction of lanthanoids(III) (Ln) with several  -diketones and trioctylphosphine oxide (TOPO) in 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide, and clarified that the separability of Ln was remarkably enhanced by the selective synergism for heavier rate earth. In this study, the extraction equilibria including several adduct formations for Nd, Eu, and Lu in the synergistic ionic-liquid extraction system with 2-thenoyltrifluoroacetone (Htta) and TOPO was investigated. The respective extraction constants of each adduct were determined from a three-dimensional non-linear analysis of log
-diketones and trioctylphosphine oxide (TOPO) in 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide, and clarified that the separability of Ln was remarkably enhanced by the selective synergism for heavier rate earth. In this study, the extraction equilibria including several adduct formations for Nd, Eu, and Lu in the synergistic ionic-liquid extraction system with 2-thenoyltrifluoroacetone (Htta) and TOPO was investigated. The respective extraction constants of each adduct were determined from a three-dimensional non-linear analysis of log  -log [tta
-log [tta ]
]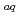 -log [TOPO]
-log [TOPO]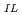 , and the formation constants of each adduct in IL were calculated. The order of the formation constants of Ln(tta)
, and the formation constants of each adduct in IL were calculated. The order of the formation constants of Ln(tta) (TOPO)
(TOPO)
 was found to be Lu
was found to be Lu  Eu
Eu  Nd. Therefore, it was indicated that the formation of hydrophobic charged ternary complexes is a factor for the selective synergism.
Nd. Therefore, it was indicated that the formation of hydrophobic charged ternary complexes is a factor for the selective synergism.
Okamura, Hiroyuki; Hirayama, Naoki*; Shimojo, Kojiro; Naganawa, Hirochika; Imura, Hisanori*
no journal, ,
no abstracts in English