Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yamaguchi, Hisato*; Ogawa, Shuichi*; Watanabe, Daiki*; Hozumi, Hideaki*; Gao, Y.*; Eda, Goki*; Mattevi, C.*; Fujita, Takeshi*; Yoshigoe, Akitaka; Ishizuka, Shinji*; et al.
Physica Status Solidi (A), 213(9), p.2380 - 2386, 2016/09
Times Cited Count:14 Percentile:51.12(Materials Science, Multidisciplinary)We report valence-band electronic structure evolution of graphene oxide (GO) upon its thermal reduction. The degree of oxygen functionalization was controlled by annealing temperature, and an electronic structure evolution was monitored using real-time ultraviolet photoelectron spectroscopy. We observed a drastic increase in the density of states around the Fermi level upon thermal annealing at  600
600 C. The result indicates that while there is an apparent bandgap for GO prior to a thermal reduction, the gap closes after an annealing around that temperature. This trend of bandgap closure was correlated with the electrical, chemical, and structural properties to determine a set of GO material properties that is optimal for optoelectronics. The results revealed that annealing at a temperature of 500
C. The result indicates that while there is an apparent bandgap for GO prior to a thermal reduction, the gap closes after an annealing around that temperature. This trend of bandgap closure was correlated with the electrical, chemical, and structural properties to determine a set of GO material properties that is optimal for optoelectronics. The results revealed that annealing at a temperature of 500 C leads to the desired properties, demonstrated by a uniform and an order of magnitude enhanced photocurrent map of an individual GO sheet compared to an as-synthesized counterpart.
C leads to the desired properties, demonstrated by a uniform and an order of magnitude enhanced photocurrent map of an individual GO sheet compared to an as-synthesized counterpart.
 Ge
Ge alloy layer studied by real-time photoelectron spectroscopy
alloy layer studied by real-time photoelectron spectroscopyOgawa, Shuichi*; Hozumi, Hideaki*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Kaga, Toshiteru*; Teraoka, Yuden; Takakuwa, Yuji*
Oyo Butsuri Gakkai Hakumaku, Hyomen Butsuri Bunkakai, Shirikon Tekunoroji Bunkakai Kyosai Tokubetsu Kenkyukai Kenkyu Hokoku, p.67 - 70, 2011/01
The oxidation-enhanced Ge atoms condensation kinetics on an Si Ge
Ge alloy layer has been investigated by the real-time photoemission spectroscopy using the synchrotron radiation. The Si
alloy layer has been investigated by the real-time photoemission spectroscopy using the synchrotron radiation. The Si Ge
Ge alloy layer was formed with a thermal evaporation method on a p-type Si(001) surface, and this alloy layer was oxidized at Langmuir-type adsorption. During oxidation at 773 KC, it is found that the Ge atoms are not oxidized, only SiO
alloy layer was formed with a thermal evaporation method on a p-type Si(001) surface, and this alloy layer was oxidized at Langmuir-type adsorption. During oxidation at 773 KC, it is found that the Ge atoms are not oxidized, only SiO film is formed on the Si
film is formed on the Si Ge
Ge alloy layer. Furthermore, the desorption of GeO molecules does not occur during the oxidation of alloy layer. On the other hand, not only Si atoms but also Ge atoms are oxidized at room temperature. This difference can be explained using the unified oxidation model mediated by the point defect generation, namely it is suggested that a lot of vacancies are generated during oxidation of the Si
alloy layer. Furthermore, the desorption of GeO molecules does not occur during the oxidation of alloy layer. On the other hand, not only Si atoms but also Ge atoms are oxidized at room temperature. This difference can be explained using the unified oxidation model mediated by the point defect generation, namely it is suggested that a lot of vacancies are generated during oxidation of the Si Ge
Ge alloy layer at 773K and Ge atoms diffuse through these vacancies.
alloy layer at 773K and Ge atoms diffuse through these vacancies.
 C
C alloy layer/Si(001) surface under oxide growth and etching conditions
alloy layer/Si(001) surface under oxide growth and etching conditionsHozumi, Hideaki*; Ogawa, Shuichi*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Harries, J.; Teraoka, Yuden; Takakuwa, Yuji*
Oyo Butsuri Gakkai Hakumaku, Hyomen Butsuri Bunkakai, Shirikon Tekunoroji Bunkakai Kyosai Tokubetsu Kenkyukai Kenkyu Hokoku, p.181 - 184, 2010/01
The oxidation kinetics on the Si C
C alloy layer has been investigated using the real-time XPS measurement. Experiments were performed at the BL23SU of SPring-8. The Si
alloy layer has been investigated using the real-time XPS measurement. Experiments were performed at the BL23SU of SPring-8. The Si C
C alloy layer was formed with exposing a p-type Si(001) surface to ethylene, and the Si
alloy layer was formed with exposing a p-type Si(001) surface to ethylene, and the Si C
C alloy layer was oxidized at Langmuir-type adsorption (773 K) and 2D oxide island growth (933 K), respectively. In case of Langmuir-type adsorption, it is found that no carbon atoms are oxidized and carbon concentration at the SiO
alloy layer was oxidized at Langmuir-type adsorption (773 K) and 2D oxide island growth (933 K), respectively. In case of Langmuir-type adsorption, it is found that no carbon atoms are oxidized and carbon concentration at the SiO /Si interface increases. These results indicate the carbon atom condensation occurs, leading to the SiO
/Si interface increases. These results indicate the carbon atom condensation occurs, leading to the SiO /Si
/Si C
C /Si layers formation. On the other hand, the carbon concentration decrease by 20% in spite of the etching of 38 Si layers in the 2D oxide island growth. Based on these results, it is found that the diffusion of carbon atoms is occurred due to not only oxide growth but also Si etching.
/Si layers formation. On the other hand, the carbon concentration decrease by 20% in spite of the etching of 38 Si layers in the 2D oxide island growth. Based on these results, it is found that the diffusion of carbon atoms is occurred due to not only oxide growth but also Si etching.
Hozumi, Hideaki*; Ogawa, Shuichi*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Harries, J.; Teraoka, Yuden; Takakuwa, Yuji*
JSPS 141 Committee Activity Report, p.317 - 322, 2009/12
It is reported that Si C
C alloy layer was formed on an Si(001) surface with C
alloy layer was formed on an Si(001) surface with C H
H exposure at 933 K before nucleation. However information of the chemical bonding state and concentration of adsorbed carbon has not been clear. Therefore the carbonization reaction kinetics on an Si(001) surface reacted with C
exposure at 933 K before nucleation. However information of the chemical bonding state and concentration of adsorbed carbon has not been clear. Therefore the carbonization reaction kinetics on an Si(001) surface reacted with C H
H exposure was observed by real-time XPS to investigate the 3C-SiC nucleation kinetics. The experiments were performed at the BL23SU of SPring-8. It is suggested that the C1s spectra is composed of at least three chemically-shifted components, which are assigned to carbon-poor Si
exposure was observed by real-time XPS to investigate the 3C-SiC nucleation kinetics. The experiments were performed at the BL23SU of SPring-8. It is suggested that the C1s spectra is composed of at least three chemically-shifted components, which are assigned to carbon-poor Si C
C alloy, carbon-rich Si
alloy, carbon-rich Si C
C alloy and 3C-SiC. The 3C-SiC nuclei began to generate at 8000s. Using C1s and Si2p
alloy and 3C-SiC. The 3C-SiC nuclei began to generate at 8000s. Using C1s and Si2p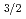 peaks, carbon concentration of the Si
peaks, carbon concentration of the Si C
C alloy layer was estimated to be about 0.17 of x. Therefore it is suggested that critical carbon concentration is 17%.
alloy layer was estimated to be about 0.17 of x. Therefore it is suggested that critical carbon concentration is 17%.
 /Si interface during oxidation of Si
/Si interface during oxidation of Si C
C alloy layer on Si(001) surfaces
alloy layer on Si(001) surfacesHozumi, Hideaki*; Ogawa, Shuichi*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Harries, J.; Teraoka, Yuden; Takakuwa, Yuji*
no journal, ,
The oxidation of Si C
C surface was studied by real-time X-ray photoelectron spectroscopy using synchrotron radiation to clarify the correlation between oxidation-induced strain and oxidation kinetics. Firstly, a p-type Si(001) surface was carbonized by C
surface was studied by real-time X-ray photoelectron spectroscopy using synchrotron radiation to clarify the correlation between oxidation-induced strain and oxidation kinetics. Firstly, a p-type Si(001) surface was carbonized by C H
H . Then, the surface was oxidized at 773 K under 5.0
. Then, the surface was oxidized at 773 K under 5.0 10
10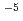 Pa of O
Pa of O . After saturation, the O
. After saturation, the O pressure was increased to 1.3
pressure was increased to 1.3 10
10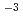 Pa. Si-2p, O-1s, and C-1s photoelectron spectra were measured alternately during oxidation. From the time evolution of C-1s/Si-2p(Si
Pa. Si-2p, O-1s, and C-1s photoelectron spectra were measured alternately during oxidation. From the time evolution of C-1s/Si-2p(Si ) ratio, it was found that the Si
) ratio, it was found that the Si composes of bulk and strained Si atoms. The increase of C-1s/Si-2p(Si
composes of bulk and strained Si atoms. The increase of C-1s/Si-2p(Si ) ratio suggests the concentration of carbon at the interface. After O
) ratio suggests the concentration of carbon at the interface. After O increase, the interface oxidation proceeds. The rate and the oxidation-induced strain decreased with oxidation. We concluded the interface oxidation rate is enhanced by oxidation-induced strain.
increase, the interface oxidation proceeds. The rate and the oxidation-induced strain decreased with oxidation. We concluded the interface oxidation rate is enhanced by oxidation-induced strain.
Hozumi, Hideaki*; Ogawa, Shuichi*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Harries, J.; Teraoka, Yuden; Takakuwa, Yuji*
no journal, ,
The 3C-SiC formation on the Si(001) surface by ethylene has been studied. The facts that 3C-SiC nucleation takes place after incuvation time and four Si C
C alloy layers are formed during the incuvation time. In this study, critical carbon concentration for SiC nucleation has been investigated. Time evolution of Si2p and C1s photoemission peaks were observed by real-time synchrotron radiation photoemission spectroscopy during exposure Si(001) substrate to ethylene gas at 913 K. Consequently, 3C-SiC nucleation takes place at carbon concentration of 15%.
alloy layers are formed during the incuvation time. In this study, critical carbon concentration for SiC nucleation has been investigated. Time evolution of Si2p and C1s photoemission peaks were observed by real-time synchrotron radiation photoemission spectroscopy during exposure Si(001) substrate to ethylene gas at 913 K. Consequently, 3C-SiC nucleation takes place at carbon concentration of 15%.
Hozumi, Hideaki*; Ogawa, Shuichi*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Harries, J.; Teraoka, Yuden; Takakuwa, Yuji*
no journal, ,
The oxidation kinetics on the Si C
C alloy layer was investigated using the real-time XPS measurement to reveal the oxidation mechanism of Si alloy layer. Experiments were performed using the surface reaction analysis apparatus placed at the BL23SU of SPring-8. The Si
alloy layer was investigated using the real-time XPS measurement to reveal the oxidation mechanism of Si alloy layer. Experiments were performed using the surface reaction analysis apparatus placed at the BL23SU of SPring-8. The Si C
C alloy layer was formed with ethylene (C
alloy layer was formed with ethylene (C H
H ) exposure to Si(001) surface and then that surface was oxidized at 963K. An oxidation rate of the Si
) exposure to Si(001) surface and then that surface was oxidized at 963K. An oxidation rate of the Si C
C alloy layer is faster than that of a clean Si surface. The C 1s photoelectron intensity decreased by 10% in spite of the etching of 45 Si layers due to SiO desorption. Based on these results, it is suggested that all C atoms diffuse into Si substrate due to oxidation-induced strain.
alloy layer is faster than that of a clean Si surface. The C 1s photoelectron intensity decreased by 10% in spite of the etching of 45 Si layers due to SiO desorption. Based on these results, it is suggested that all C atoms diffuse into Si substrate due to oxidation-induced strain.
Watanabe, Daiki*; Ogawa, Shuichi*; Yamaguchi, Hisato*; Hozumi, Hideaki*; Mattevi, C.*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Teraoka, Yuden; Yamada, Takatoshi*; Chhowalla, M.*; et al.
no journal, ,
Ogawa, Shuichi*; Hozumi, Hideaki*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Teraoka, Yuden; Takakuwa, Yuji*
no journal, ,
In this study, real-time XPS observation of Si(001) surface oxidation is employed to investigate the surface oxidation reaction manner dependence of SiO /Si interface and growth rate of the second oxide layer. Oxidation experiments were performed at the surface reaction analysis apparatus placed at BL23SU, SPring-8. A p-type Si(001) surface was oxidized by dry oxygen. In the Langmuir-type adsorption, the amount of strained Si atoms at the end of surface oxidation is 0.46 ML, and then significant interface oxidation can be observed. On the other hand, in the 2D oxide island growth, the amount of strained Si atoms is 0.15 ML and progress of interface oxidation hardly observed. These results indicate that when interface strain is large, the interface oxidation rate also large, and then that strongly depends on Si surface oxidation manner.
/Si interface and growth rate of the second oxide layer. Oxidation experiments were performed at the surface reaction analysis apparatus placed at BL23SU, SPring-8. A p-type Si(001) surface was oxidized by dry oxygen. In the Langmuir-type adsorption, the amount of strained Si atoms at the end of surface oxidation is 0.46 ML, and then significant interface oxidation can be observed. On the other hand, in the 2D oxide island growth, the amount of strained Si atoms is 0.15 ML and progress of interface oxidation hardly observed. These results indicate that when interface strain is large, the interface oxidation rate also large, and then that strongly depends on Si surface oxidation manner.
Takakuwa, Yuji*; Ogawa, Shuichi*; Hozumi, Hideaki*; Kawamura, Tomofumi*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Teraoka, Yuden
no journal, ,
In order to investigate the surface reaction kinetics on Si C
C alloy layer, the carbonization reaction on Si(001) surface by ethylene using the real-time XPS observation by synchrotron radiation. Experiments were performed at the surface reaction analysis apparatus placed at BL23SU, SPring-8. A p-type Si(001) surface heated as 913 K was carbonized using C
alloy layer, the carbonization reaction on Si(001) surface by ethylene using the real-time XPS observation by synchrotron radiation. Experiments were performed at the surface reaction analysis apparatus placed at BL23SU, SPring-8. A p-type Si(001) surface heated as 913 K was carbonized using C H
H diluted by He. It is found from Si 2p photoelectron spectra that Si-C bounding component increases in proportion as dosage of C
diluted by He. It is found from Si 2p photoelectron spectra that Si-C bounding component increases in proportion as dosage of C H
H , and same tendency is observed from dosage dependence of C 1s photoelectron intensity. These results indicate that the dissociative adsorption of C
, and same tendency is observed from dosage dependence of C 1s photoelectron intensity. These results indicate that the dissociative adsorption of C H
H on Si(001) surface is zero-order reaction, and the activation energy of diffusion of adsorbed C atoms into Si subsurface is very small.
on Si(001) surface is zero-order reaction, and the activation energy of diffusion of adsorbed C atoms into Si subsurface is very small.
 /Si interfacial oxidation, 2; Carbon diffusion during 2D oxide island growth
/Si interfacial oxidation, 2; Carbon diffusion during 2D oxide island growthHozumi, Hideaki*; Ogawa, Shuichi*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Harries, J.; Teraoka, Yuden; Takakuwa, Yuji*
no journal, ,
The oxidation reaction of Si C
C alloy layer which was formed with C
alloy layer which was formed with C H
H exposure on a p-type Si(001) surface was observed by real-time XPS to investigate the role of lattice strain. Experiments were performed using the surface reaction analysis apparatus placed at the BL23SU of SPring-8. When Si
exposure on a p-type Si(001) surface was observed by real-time XPS to investigate the role of lattice strain. Experiments were performed using the surface reaction analysis apparatus placed at the BL23SU of SPring-8. When Si C
C alloy layer was oxidized at 660
alloy layer was oxidized at 660  C, oxidation mode was 2D oxide island growth. Etching advanced as well as oxidation films growth. Nearly 45 layers were etched for 7000 s from oxidation start regardless of oxide coverage was nearly 3 percents. This suggested SiO desorption occurred. In this result, Si
C, oxidation mode was 2D oxide island growth. Etching advanced as well as oxidation films growth. Nearly 45 layers were etched for 7000 s from oxidation start regardless of oxide coverage was nearly 3 percents. This suggested SiO desorption occurred. In this result, Si C
C alloy layer that was formed on surface 4th layers should be completely rejected, but C 1s intensity that was normalized by Si 2p bulk intensity was constant. Therefore it was suggested carbon atoms diffused into Si substrate in SiO desorption.
alloy layer that was formed on surface 4th layers should be completely rejected, but C 1s intensity that was normalized by Si 2p bulk intensity was constant. Therefore it was suggested carbon atoms diffused into Si substrate in SiO desorption.
 formation
formationHozumi, Hideaki*; Ogawa, Shuichi*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Teraoka, Yuden; Takakuwa, Yuji*
no journal, ,
In order to make clear Ge concentration processes during oxidation of an SiGe alloy layer, Ge3d photoelectron spectra were observed during oxidation of an Si Ge
Ge alloy layer using real-time XPS. All experiments were conducted using the surface reaction analysis apparatus at BL23SU in the SPring-8. A Ge layer was formed on the p-type Si(001) substrate with about 2 ML thickness by evapolation. The layer was annealed up to 993 K to form an Si
alloy layer using real-time XPS. All experiments were conducted using the surface reaction analysis apparatus at BL23SU in the SPring-8. A Ge layer was formed on the p-type Si(001) substrate with about 2 ML thickness by evapolation. The layer was annealed up to 993 K to form an Si Ge
Ge alloy layer. The surface was oxidized in the O
alloy layer. The surface was oxidized in the O ambient of 5.0
ambient of 5.0 10
10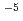 Pa for 6400 s, and then further oxidation was made at 1.3
Pa for 6400 s, and then further oxidation was made at 1.3 10
10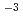 Pa for 5300 s. The surface temperature was room temperature and 773 K. In the case of 773 K, Ge atoms were not oxidized whereas Si atoms were selectively oxidized. The oxidation rate was faster than that of an Si(001) surface. These facts reveal that Ge atoms diffuse into the Si substrate due to SiO
Pa for 5300 s. The surface temperature was room temperature and 773 K. In the case of 773 K, Ge atoms were not oxidized whereas Si atoms were selectively oxidized. The oxidation rate was faster than that of an Si(001) surface. These facts reveal that Ge atoms diffuse into the Si substrate due to SiO layer formation.
layer formation.
Hozumi, Hideaki*; Ogawa, Shuichi*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Harries, J.; Teraoka, Yuden; Takakuwa, Yuji*
no journal, ,
The oxidation of SiGe alloy layer is used for the concentration process of Ge in the CMOS devices. In this study, the oxidation reaction kinetics on SiGe alloy layers was investigated by real-time XPS to reveal the oxidation and Ge concentration kinetics. Experiments were performed at the BL23SU of SPring-8. Ge was deposited on the Si(001) surface at room temperature. After deposition, the sample was annealed at 933 K for 20 min. and then oxidized using oxygen gas at 773 K. The O 1s, Si 2p and Ge 3d photoelectron spectra were measured during oxidation. O 1s photoelectron intensity increased rapidly and then saturate after 2000 s. The oxidation rate on the SiGe alloy surface was slower than that of the Si clean surface. However, SiO component on the SiGe alloy surface increased. It was found that Ge was not oxidized. These results indicate that Ge atoms diffuse into Si substrate. This Ge diffusion may be caused by the oxidation-induced strain.
component on the SiGe alloy surface increased. It was found that Ge was not oxidized. These results indicate that Ge atoms diffuse into Si substrate. This Ge diffusion may be caused by the oxidation-induced strain.
 formation during thermal oxidation of Si
formation during thermal oxidation of Si Ge
Ge alloy layer on Si(001) surfaces
alloy layer on Si(001) surfacesHozumi, Hideaki*; Ogawa, Shuichi*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Harries, J.; Teraoka, Yuden; Takakuwa, Yuji*
no journal, ,
In order to clarify the concentration mechanism of Ge atoms for strained-Si CMOS devices, the oxidation reaction kinetics of Si Ge
Ge alloy layer was observed by real-time XPS. By comparison of the oxidation reaction kinetics on the Si
alloy layer was observed by real-time XPS. By comparison of the oxidation reaction kinetics on the Si Ge
Ge alloy layer among two oxidation temperature (room temperature and 773 K), it was found that Ge was significantly oxidized at room temperature, although SiO
alloy layer among two oxidation temperature (room temperature and 773 K), it was found that Ge was significantly oxidized at room temperature, although SiO was exclusively formed at 773 K. This result indicates that the oxidation rate of Ge atoms depends of the diffusion of Ge atoms due to oxidation-induced strain.
was exclusively formed at 773 K. This result indicates that the oxidation rate of Ge atoms depends of the diffusion of Ge atoms due to oxidation-induced strain.
Hozumi, Hideaki*; Kaga, Toshihide*; Ogawa, Shuichi*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Teraoka, Yuden; Takakuwa, Yuji*
no journal, ,
In order to clarify the difference of the oxidation reaction mechanism between an Si Ge
Ge and Si
and Si C
C alloy layer, the real time photoelectron spectroscopy was employed for observing the oxidation rate and behavior of Ge and C atoms during oxidation. The experiments were performed using the surface reaction analysis apparatus placed at the BL23SU of SPring-8. By comparing between the both alloy surfaces, it is found that initial oxidation rate on the SiGe alloy was slower than that on the SiC alloy. Here, only Si atoms were oxidized on the both alloy surfaces. C atoms were condensed at SiO
alloy layer, the real time photoelectron spectroscopy was employed for observing the oxidation rate and behavior of Ge and C atoms during oxidation. The experiments were performed using the surface reaction analysis apparatus placed at the BL23SU of SPring-8. By comparing between the both alloy surfaces, it is found that initial oxidation rate on the SiGe alloy was slower than that on the SiC alloy. Here, only Si atoms were oxidized on the both alloy surfaces. C atoms were condensed at SiO /Si interface and 3C-SiC alloy were formed. Ge atoms were diffused into Si substrate. These facts are resulted in the oxidation-strain-induced point-defect generation, in which C and Ge atoms are exchanged for Si atoms through the vacancies.
/Si interface and 3C-SiC alloy were formed. Ge atoms were diffused into Si substrate. These facts are resulted in the oxidation-strain-induced point-defect generation, in which C and Ge atoms are exchanged for Si atoms through the vacancies.
Hozumi, Hideaki*; Yamaguchi, Hisato*; Kaga, Toshihide*; Eda, Goki*; Mattevi, C.*; Ogawa, Shuichi*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Teraoka, Yuden; Yamada, Takatoshi*; et al.
no journal, ,
In order to clarify the time evolution of the chemical bonding state during thermal reduction of graphene oxide (GO), real-time photoelectron spectroscopy was employed for observing the thermal reduction kinetics of GO. The GO was prepared by the modified Hummer method. The experiments were performed using the surface reaction analysis apparatus placed at the BL23SU of SPring-8. The XPS measurements were performed simultaneously during the annealing at 473 K, 673 K, 873 K, and 1073 K. The C1s photoelectron spectra are decomposed by 8 components. The 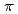 -
-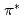 transition loss peak intensity is propotional to the intensity of sp
transition loss peak intensity is propotional to the intensity of sp graphene components with temperature elevation. In addition, defect intensity increased in proportion with the sp
graphene components with temperature elevation. In addition, defect intensity increased in proportion with the sp graphene intensity. These facts indicate that defects were formed on the graphene during reduction and these defects cause the recovery of electric conductivity, that is, the appearance of Fermi edge.
graphene intensity. These facts indicate that defects were formed on the graphene during reduction and these defects cause the recovery of electric conductivity, that is, the appearance of Fermi edge.
Ogawa, Shuichi*; Yamaguchi, Hisato*; Hozumi, Hideaki*; Kaga, Toshihide*; Eda, Goki*; Mattevi, C.*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Teraoka, Yuden; Yamada, Takatoshi*; et al.
no journal, ,
no abstracts in English
Ogawa, Shuichi*; Yamada, Takatoshi*; Ishizuka, Shinji*; Yoshigoe, Akitaka; Kaga, Toshiteru*; Hozumi, Hideaki*; Hasegawa, Masataka*; Teraoka, Yuden; Takakuwa, Yuji*
no journal, ,
Watanabe, Daiki*; Ogawa, Shuichi*; Yamaguchi, Hisato*; Hozumi, Hideaki*; Eda, Goki*; Mattevi, C.*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Teraoka, Yuden; Yamada, Takatoshi*; et al.
no journal, ,
no abstracts in English
Watanabe, Daiki*; Ogawa, Shuichi*; Yamaguchi, Hisato*; Hozumi, Hideaki*; Eda, Goki*; Mattevi, C.*; Yoshigoe, Akitaka; Ishizuka, Shinji*; Teraoka, Yuden; Yamada, Takatoshi*; et al.
no journal, ,
no abstracts in English