Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 mixed oxides
mixed oxidesVauchy, R.; Matsumoto, Taku; Hirooka, Shun; Uno, Hiroki*; Tamura, Tetsuya*; Arima, Tatsumi*; Inagaki, Yaohiro*; Idemitsu, Kazuya*; Nakamura, Hiroki; Machida, Masahiko; et al.
Journal of Nuclear Materials, 588, p.154786_1 - 154786_13, 2024/01
Times Cited Count:6 Percentile:84.29(Materials Science, Multidisciplinary) Ln
Ln O
O (Ln = Gd, Er) solid solution
(Ln = Gd, Er) solid solutionPham, V. M.*; Arima, Tatsumi*; Inagaki, Yaohiro*; Idemitsu, Kazuya*; Akiyama, Daisuke*; Nagai, Takayuki; Okamoto, Yoshihiro
Journal of Nuclear Materials, 556, p.153189_1 - 153189_9, 2021/12
Times Cited Count:1 Percentile:9.64(Materials Science, Multidisciplinary)The crystal structure change was evaluated for (1-x)UO -xLnO
-xLnO (Ln=Gd or Er; x = 0 to 0.4) samples sintered at 1973 K for 8 h under Ar and Ar-10%H
(Ln=Gd or Er; x = 0 to 0.4) samples sintered at 1973 K for 8 h under Ar and Ar-10%H atmospheres. The effect of LnO
atmospheres. The effect of LnO doping on the crystal structure of UO
doping on the crystal structure of UO was investigated by X-ray diffraction (XRD) and X-ray absorption fine structure (XAFS). LnO
was investigated by X-ray diffraction (XRD) and X-ray absorption fine structure (XAFS). LnO doping into UO
doping into UO reduced the lattice parameter of UO
reduced the lattice parameter of UO -LnO
-LnO solid solutions up to 40mol% LnO
solid solutions up to 40mol% LnO . The lattice parameters of these samples were comparable to those of stoichiometric (U,Ln)O
. The lattice parameters of these samples were comparable to those of stoichiometric (U,Ln)O solid solutions, that is, the O/M ratios were close to 2.00. The U L
solid solutions, that is, the O/M ratios were close to 2.00. The U L -edge XANES analysis showed that higher U oxidation states of +5 or +6 formed, in addition to + 4. The EXAFS analysis indicated that the interatomic distances of U-O and Gd-O decreased with increasing x, whereas those of Er-O may not decrease monotonically.
-edge XANES analysis showed that higher U oxidation states of +5 or +6 formed, in addition to + 4. The EXAFS analysis indicated that the interatomic distances of U-O and Gd-O decreased with increasing x, whereas those of Er-O may not decrease monotonically.
Arima, Tatsumi*; Idemitsu, Kazuya*; Inagaki, Yaohiro*; Kawamura, Katsuyuki*; Tachi, Yukio; Yotsuji, Kenji
Progress in Nuclear Energy, 92, p.286 - 297, 2016/09
Times Cited Count:15 Percentile:77.12(Nuclear Science & Technology)Diffusion and adsorption behavior of uranyl (UO ) species is important for the performance assessment of radioactive waste disposal. The diffusion behaviors of UO
) species is important for the performance assessment of radioactive waste disposal. The diffusion behaviors of UO , K
, K , CO
, CO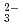 and Cl
and Cl and H
and H O in the aqueous solutions were evaluated by molecular dynamics (MD) calculations. The diffusion coefficient (De) of UO
O in the aqueous solutions were evaluated by molecular dynamics (MD) calculations. The diffusion coefficient (De) of UO is the smallest and is 26% less than the self-diffusion coefficient of H
is the smallest and is 26% less than the self-diffusion coefficient of H O. For the aqueous solution with high concentration of carbonate ions, uranyl carbonate complexes: UO
O. For the aqueous solution with high concentration of carbonate ions, uranyl carbonate complexes: UO CO
CO and UO
and UO (CO
(CO )
) can be observed. For the clay (montmorillonite or illite)-aqueous solution systems, the adsorption and diffusion behaviors of UO
can be observed. For the clay (montmorillonite or illite)-aqueous solution systems, the adsorption and diffusion behaviors of UO and K
and K were evaluated by MD calculations. The distribution coefficients (Kd) increase with the layer charge of clay, and Kd of UO
were evaluated by MD calculations. The distribution coefficients (Kd) increase with the layer charge of clay, and Kd of UO might be smaller than that of K
might be smaller than that of K . Further, their two-dimensional diffusion coefficients were relatively small in the adsorption layer and were extremely small for illite with higher layer charge.
. Further, their two-dimensional diffusion coefficients were relatively small in the adsorption layer and were extremely small for illite with higher layer charge.
Miwa, Shuhei; Osaka, Masahiko; Nozaki, Takahiro*; Arima, Tatsumi*; Idemitsu, Kazuya*
Journal of Nuclear Materials, 465, p.840 - 842, 2015/10
Times Cited Count:1 Percentile:8.75(Materials Science, Multidisciplinary)Oxygen potential of a prototypic Mo-cermet fuel containing PuO was experimentally determined. It was shown that the oxygen potentials of Mo-cermet fuel containing PuO
was experimentally determined. It was shown that the oxygen potentials of Mo-cermet fuel containing PuO were the same as those of pure PuO
were the same as those of pure PuO . It was also confirmed that the gradual oxidation of the Mo matrix occurred only above the oxygen potential of Mo/MoO
. It was also confirmed that the gradual oxidation of the Mo matrix occurred only above the oxygen potential of Mo/MoO . It is concluded that the oxidation-reduction behavior of the Mo-cermet fuel can be evaluated individually for each phase of actinides oxide and Mo matrix. Better phase structures of the Mo-cermet fuel for taking full advantage of the oxidation-reduction controllability were suggested by the confinement of the actinides oxide phase with Mo.
. It is concluded that the oxidation-reduction behavior of the Mo-cermet fuel can be evaluated individually for each phase of actinides oxide and Mo matrix. Better phase structures of the Mo-cermet fuel for taking full advantage of the oxidation-reduction controllability were suggested by the confinement of the actinides oxide phase with Mo.
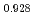 Am
Am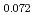 )O
)O at high temperatures
at high temperaturesMatsumoto, Taku; Arima, Tatsumi*; Inagaki, Yaohiro*; Idemitsu, Kazuya*; Kato, Masato; Morimoto, Kyoichi; Sunaoshi, Takeo*
Journal of Nuclear Science and Technology, 52(10), p.1296 - 1302, 2015/10
Times Cited Count:11 Percentile:63.64(Nuclear Science & Technology)The oxygen potentials of (Pu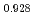 Am
Am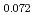 )O
)O were measured at 1873K, 1773K and 1473K by gas equilibrium method. It was shown that following the reduction of Am at the O/M ratio above 1.96, Pu was reduced at the O/M ratio below 1.96.
were measured at 1873K, 1773K and 1473K by gas equilibrium method. It was shown that following the reduction of Am at the O/M ratio above 1.96, Pu was reduced at the O/M ratio below 1.96.
Maeda, Toshikatsu; Watanabe, Koichi; Omori, Hiroyuki*; Sakamaki, Keiko; Inagaki, Yaohiro*; Idemitsu, Kazuya*
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 21(2), p.63 - 74, 2014/12
Static leach tests were conducted for simulated HLW glass in CaCl /Ca(OH)
/Ca(OH) solutions to investigate the corrosion behavior of HLW glass under calcium-rich environments induced by cement based materials in geological repositories. Another series of leach tests were conducted in deionized water in the presence of iron to investigate the effects of iron over-pack on the glass corrosion. In Ca solutions, corrosion of the glass was inhibited during the test period compared to that in deionized water, while the corrosion was enhanced at the presence of iron. The enhancement of the glass corrosion was assumed to be accompanied with transformation of silica, a glass network former, into iron silicates.
solutions to investigate the corrosion behavior of HLW glass under calcium-rich environments induced by cement based materials in geological repositories. Another series of leach tests were conducted in deionized water in the presence of iron to investigate the effects of iron over-pack on the glass corrosion. In Ca solutions, corrosion of the glass was inhibited during the test period compared to that in deionized water, while the corrosion was enhanced at the presence of iron. The enhancement of the glass corrosion was assumed to be accompanied with transformation of silica, a glass network former, into iron silicates.
Sato, Isamu; Arima, Tatsumi*; Nishina, Masahiro*; Tanaka, Kosuke; Onose, Shoji; Idemitsu, Kazuya*
JAEA-Research 2012-006, 66 Pages, 2012/05
As one of the important properties for fuel manufacturability and burning behavior, the diffusion behavior of actinides in oxide fuels was investigated by both the experimental and the molecular dynamics simulation (MD). Using diffusion couples consisted of an Am containing mixed oxide fuel and a UO fuel, the diffusion tests were performed. The diffusion coefficients were estimated to be 10
fuel, the diffusion tests were performed. The diffusion coefficients were estimated to be 10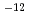 m
m /s
/s  10
10 m
m /s. In addition, the difference between Pu and Am diffusion coefficients was vanishingly small. The temperature dependence of bulk diffusion coefficients of actinides in mixed oxide fuels could be evaluated by MD. An evaluation technique for the grain boundary diffusion could be established based on the coincidence site lattice theory. The practical diffusion coefficients were obtained by combining data from the experiments with those predicted from MD. The practical diffusion coefficients obtained was discussed for use of a fuel behavior analysis code.
/s. In addition, the difference between Pu and Am diffusion coefficients was vanishingly small. The temperature dependence of bulk diffusion coefficients of actinides in mixed oxide fuels could be evaluated by MD. An evaluation technique for the grain boundary diffusion could be established based on the coincidence site lattice theory. The practical diffusion coefficients were obtained by combining data from the experiments with those predicted from MD. The practical diffusion coefficients obtained was discussed for use of a fuel behavior analysis code.
Inagaki, Yaohiro*; Makigaki, Hikaru; Idemitsu, Kazuya*; Arima, Tatsumi*; Mitsui, Seiichiro; Noshita, Kenji*
Journal of Nuclear Science and Technology, 49(4), p.438 - 449, 2012/04
Times Cited Count:20 Percentile:78.78(Nuclear Science & Technology)Dissolution tests were performed for a simulated HLW glass by using a Micro-Channel Flow-Through (MCFT) test to evaluate the initial dissolution rate, 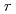
 , as a function of pH and temperature. The results indicated that the
, as a function of pH and temperature. The results indicated that the 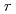
 shows a "V-shaped" pH dependence at 25
shows a "V-shaped" pH dependence at 25 C, which is almost consistent with the previous results measured by using other test methods including Single Pass Flow-Through (SPFT) test. At elevated temperatures, however, the
C, which is almost consistent with the previous results measured by using other test methods including Single Pass Flow-Through (SPFT) test. At elevated temperatures, however, the 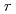
 shows a "U-shaped" pH dependence with a flat bottom at neutral pH, which differs from the previous results. The results also indicated that the MCFT provided a higher value of the
shows a "U-shaped" pH dependence with a flat bottom at neutral pH, which differs from the previous results. The results also indicated that the MCFT provided a higher value of the 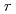
 with a steep slope of pH dependence than the SPFT results at basic pH from 8 to 11 at 90
with a steep slope of pH dependence than the SPFT results at basic pH from 8 to 11 at 90 C. With respect to the temperature dependence, the
C. With respect to the temperature dependence, the 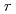
 increases with temperature according to an Arrhenius law at any pH, and the apparent activation energy increases with pH, which suggests that the dissolution mechanism can change depending on pH.
increases with temperature according to an Arrhenius law at any pH, and the apparent activation energy increases with pH, which suggests that the dissolution mechanism can change depending on pH.
Yamagishi, Isao; Mimura, Hitoshi*; Idemitsu, Kazuya*
Nihon Genshiryoku Gakkai-Shi ATOMO , 54(3), p.166 - 170, 2012/03
, 54(3), p.166 - 170, 2012/03
no abstracts in English
Minato, Kazuo; Konashi, Kenji*; Yamana, Hajimu*; Yamanaka, Shinsuke*; Nagasaki, Shinya*; Ikeda, Yasuhisa*; Sato, Seichi*; Arita, Yuji*; Idemitsu, Kazuya*; Koyama, Tadafumi*
Proceedings of International Conference on Toward and Over the Fukushima Daiichi Accident (GLOBAL 2011) (CD-ROM), 5 Pages, 2011/12
Actinide science research is indispensable to maintain sustainable development of innovative nuclear technology. For actinide science research, special facilities with containment and radiation shields are needed to handle actinide materials. The number of facilities for actinide science research has been decreased, especially in universities, due to the high maintenance cost. J-ACTINET was established in 2008 to promote and facilitate actinide science research and to foster many of young scientists and engineers in actinide science. The research program was carried out, through which young researchers were expected to learn how to make experiments with advanced experimental tools and to broaden their horizons. The summer schools and computational science school were held to provide students and young researchers with the opportunities to come into contact with actinide science research. The overseas dispatch program was also carried out.
Makigaki, Hikaru*; Inagaki, Yaohiro*; Idemitsu, Kazuya*; Arima, Tatsumi*; Mitsui, Seiichiro; Bamba, Tsunetaka; Noshita, Kenji*
Materials Research Society Symposium Proceedings, Vol.1193, p.307 - 314, 2009/05
By using micro-reactor flow-through test method we measured the initial dissolution rate of P0798 glass at 25 C as a function of pH between 5.6 and 12. The results showed that the initial dissolution rate determined by dissolution rate of Si has "V-shaped" pH dependency similar to R7T7 glass reported by CEA, France. We also measured the initial dissolution rate at pH 5.6 as a function of temperature between 25 and 90
C as a function of pH between 5.6 and 12. The results showed that the initial dissolution rate determined by dissolution rate of Si has "V-shaped" pH dependency similar to R7T7 glass reported by CEA, France. We also measured the initial dissolution rate at pH 5.6 as a function of temperature between 25 and 90 C, and the activation energy was evaluated to be 51 kJ/mol, which value is slightly smaller than that of R7T7 glass at pH 9 reported by CEA. On the basis of these results and comparison, we discussed the dissolution kinetics of P0798 glass.
C, and the activation energy was evaluated to be 51 kJ/mol, which value is slightly smaller than that of R7T7 glass at pH 9 reported by CEA. On the basis of these results and comparison, we discussed the dissolution kinetics of P0798 glass.
Inagaki, Yaohiro*; Mitsui, Seiichiro; Makigaki, Hikaru*; Idemitsu, Kazuya*; Arima, Tatsumi*; Bamba, Tsunetaka; Noshita, Kenji*
Materials Research Society Symposium Proceedings, Vol.1193, p.219 - 228, 2009/05
We developed a new flow-through test method using micro-reactor, and applied it to measurement of the dissolution/alteration kinetics for a simulated HLW glass (P0798). In this method, a glass coupon is placed just on a Teflon plate having a micro-channel, and a solution is injected into the inlet of micro-channel by micro-syringe pump at a constant flow rate. The injected solution flows through the micro-channel reacting with the glass to the outlet, and the outlet solution is retrieved at certain intervals to be analyzed for determination of the dissolution/alteration rate. This method has some major features, i.e., simple test apparatus with compact size, high S/V ratio, sensitive/precise measurement of the glass dissolution/alteration rate, adequate glass shape for analysis of reacted glass surface, and so on. By use of this method the dissolution/alteration rate for P0798 was measured as a function of pH, temperature, flow rate, and time, and some available results were obtained to evaluate the dissolution/alteration kinetics.
Ishidera, Takamitsu; Xia, X.*; Idemitsu, Kazuya*; Kikuchi, Yoshio*
Journal of Nuclear Science and Technology, 45(8), p.763 - 772, 2008/08
Times Cited Count:6 Percentile:38.69(Nuclear Science & Technology)For safety assessment of high-level radioactive waste disposal, it is important to identify the forms of corrosion products migrating from overpack material into compacted bentonite. In this study, the carbon steel, which was previously corroded electrochemically under aerobic condition, was in contact with compacted Kunigel V1 and Kunipia F under reducing condition for 3-4 years at room temperature. The corrosion products migrating from carbon steel into compacted bentonites were investigated by the selective dissolution analysis, which can estimate the crystallinity of Fe-bearing compounds. The valence of iron in corrosion products was investigated spectrophotometrically. Furthermore, the alteration of smectite contained in compacted bentonite to Fe-smectite was analyzed by X-ray diffractometry (XRD). The results of selective dissolution and valence analysis suggested that the corrosion products in compacted bentonite were amorphous, non-crystalline or poorly ordered Fe(OH) and Fe(OH)
and Fe(OH) . In the XRD profiles, no diffraction peak suggesting the existence of Fe-smectite in the compacted bentonite was found. Therefore, the corrosion products in compacted bentonite were considered to have no effect on the alteration of smectite contained in Kunigel V1 and Kunipia F to Fe-smectite.
. In the XRD profiles, no diffraction peak suggesting the existence of Fe-smectite in the compacted bentonite was found. Therefore, the corrosion products in compacted bentonite were considered to have no effect on the alteration of smectite contained in Kunigel V1 and Kunipia F to Fe-smectite.
Tsujita, Yuichi*; Arima, Tatsumi*; Idemitsu, Kazuya*; Suzuki, Yoshio; Kimura, Hideo
Proceedings of 16th International Conference on Nuclear Engineering (ICONE-16) (CD-ROM), 9 Pages, 2008/05
Tsujita, Yuichi*; Arima, Tatsumi*; Idemitsu, Kazuya*; Suzuki, Yoshio; Kimura, Hideo
Joho Shori Gakkai Kenkyu Hokoku 2008-ARC-177, 2008-HPC-114, p.103 - 108, 2008/03
Recently, many research works for Pu-recycle have been done. We proposed a molecular dynamics method to have deep understandings in nuclear materials. We adopted a parallel computing technique by using an ITBL's computing environment in our simulation program because a huge amount of computations and time steps is required. We received a request from application users to incorporate a user friendly interface in a computing environment for specifying parameters in a simulation program and successive operations of parallel computation and visualization on a user's terminal. So, we have built a GUI computing environment to realize such the functionalities by using an ITBL's client API. In this paper, we report objectives of this development, implementation method of this application, and examples of the computing environment. Finally, we mention its future plan.
Tsujita, Yuichi*; Arima, Tatsumi*; Idemitsu, Kazuya*; Nakajima, Norihiro; Suzuki, Yoshio; Kimura, Hideo
Kinki Daigaku Kogakubu Kenkyu Hokoku, (41), p.87 - 92, 2007/12
no abstracts in English
Arima, Tatsumi*; Idemitsu, Kazuya*; Xia, X.; Ishidera, Takamitsu; Iijima, Kazuki
JNC TY8400 2004-005, 59 Pages, 2004/05
For the safety assessment of high level waste disposal, it is necessary to investigate the radionuclide migration in the presence of corrosion products of carbon steel under reducing condition. In this study, the reliable apparent diffusion coefficients of neptunium (Np) in bentonite were obtained by in-diffusion method and migration of the corrosion products was investigated. Furthermore, effects of the corrosion of carbon steel on Np diffusion were discussed. The corrosion of carbon steel under reducing condition provided Fe ions, which were considered to migrate in the interlayer spaces of montmorillonite sheets of bentonite exchanging with two Na
ions, which were considered to migrate in the interlayer spaces of montmorillonite sheets of bentonite exchanging with two Na ions. The corrosion rate of carbon steel was controlled by the diffusivity of Fe
ions. The corrosion rate of carbon steel was controlled by the diffusivity of Fe ions into bentonite when they were accumulated at the surface of carbon steel. The corrosion rate increased with increasing dry density of bentonite because of the increase of the diffusivity of Fe
ions into bentonite when they were accumulated at the surface of carbon steel. The corrosion rate increased with increasing dry density of bentonite because of the increase of the diffusivity of Fe ions. The corrosion rate was estimated to be ~0.1 micro m/y, which was remarkable lower than the setting value, 20 micro m/y, in the second progress report. The Np profiles in the bentonite consisted of two overlapping slopes, a fast and a slow fractions, for both the experiments with and without carbon steel. The apparent diffusion coefficients of the fast and the slow fraction of Np were 10
ions. The corrosion rate was estimated to be ~0.1 micro m/y, which was remarkable lower than the setting value, 20 micro m/y, in the second progress report. The Np profiles in the bentonite consisted of two overlapping slopes, a fast and a slow fractions, for both the experiments with and without carbon steel. The apparent diffusion coefficients of the fast and the slow fraction of Np were 10 ~10
~10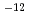 m
m /s and 10
/s and 10 ~10
~10 m
m /s, which were considered to be the diffusion of Np(V) and Np(IV), respectively. The corrosion of carbon steel provided strong reducing condition to keep most Np in the low oxidation state, Np(IV), which has lower solubility and mobility than Np(V). Therefore, it could be expected that the corrosion of carbon steel will restrain effectively migration of Np into the bentonite.
/s, which were considered to be the diffusion of Np(V) and Np(IV), respectively. The corrosion of carbon steel provided strong reducing condition to keep most Np in the low oxidation state, Np(IV), which has lower solubility and mobility than Np(V). Therefore, it could be expected that the corrosion of carbon steel will restrain effectively migration of Np into the bentonite.
Idemitsu, Kazuya*; Inagaki, Yaohiro*; Arima, Tatsumi*
JNC TY8400 2003-001, 72 Pages, 2003/05
None
Sato, Isamu; Namekawa, Takushi; ; Idemitsu, Kazuya*; ; Inagaki, Yaohiro*
Journal of Nuclear Materials, (304), p.21 - 28, 2002/00
Times Cited Count:17 Percentile:70.43(Materials Science, Multidisciplinary)None
Inagaki, Yaohiro*; Idemitsu, Kazuya*; Arima, Tatsumi*; Maeda, Toshikatsu; Ogawa, Hiromichi; Itonaga, Fumio
Materials Research Society Symposium Proceedings, Vol.713, p.589 - 596, 2002/00
A large number of studies on HLW glass corrosion have shown that the glass reacts with water to form more stable mineral phases (alteration phases) during the long-term geological disposal. The phase formation is essential to evaluate the radionuclide release from the glass during the long-term disposal. The purpose of this study is to evaluate, experimentally, the mineral phase formation from HLW glass and the associated cesium release. Static corrosion tests were performed on powdered R7T7 glass in alkalline solutions at elevated temperatures to accelerate the reaction, and mineral phases formed were analyzed by XRD. The results showed that analcime (zeolite) is formed as the dominant phase coexisting with SiO (am), and beidellite(smectite) or gibbsite coexists dependiting on the conditions. The solution analysis indicated that most of the cesium is retained in the phases of beidellite and analcime by sorption.
(am), and beidellite(smectite) or gibbsite coexists dependiting on the conditions. The solution analysis indicated that most of the cesium is retained in the phases of beidellite and analcime by sorption.