Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 SO
SO /HNO
/HNO mixed solution
mixed solutionLi, Z.*; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Kikuchi, Takahiro; Nagame, Yuichiro; Sch del, M.; Pershina, V.*; et al.
del, M.; Pershina, V.*; et al.
Radiochimica Acta, 100(3), p.157 - 164, 2012/03
Times Cited Count:14 Percentile:68.81(Chemistry, Inorganic & Nuclear) SO
SO solutions
solutionsIkarashi, Satoshi*; Sueki, Keisuke*; Tsukada, Kazuaki; Nagame, Yuichiro
no journal, ,
The adsorption rates of Zr and Hf on the cation-exchange resin in H SO
SO solutions were investigated in order to lead to the quantitative discussion of the chromatography. It was obtained the adsorption rates of
solutions were investigated in order to lead to the quantitative discussion of the chromatography. It was obtained the adsorption rates of  Zr and
Zr and 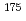 Hf on the cation-exchange resin in 0.1 M H
Hf on the cation-exchange resin in 0.1 M H SO
SO at 15, 25 and 45
at 15, 25 and 45 C as functions of the shaking times. The results showed that the equilibrium of cation-exchange was reached at 70
C as functions of the shaking times. The results showed that the equilibrium of cation-exchange was reached at 70 80 seconds and that the adsorption rates on the cation-exchange resin were not changed between 15
80 seconds and that the adsorption rates on the cation-exchange resin were not changed between 15 45
45 C in the present condition. In H
C in the present condition. In H SO
SO /HNO
/HNO mixed solution ([H
mixed solution ([H ]
]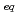 = 1.0 M), the adsorption rate was similar to those of
= 1.0 M), the adsorption rate was similar to those of  Zr and
Zr and 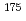 Hf on the cation-exchange resin in H
Hf on the cation-exchange resin in H SO
SO solution.
solution.
 SO
SO /HNO
/HNO mixed solution ([H
mixed solution ([H ] = 1.0 M)
] = 1.0 M)Li, Z.; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Sch del, M.*; Nagame, Yuichiro; Liang, X. H.*; Kasamatsu, Yoshitaka*; et al.
del, M.*; Nagame, Yuichiro; Liang, X. H.*; Kasamatsu, Yoshitaka*; et al.
no journal, ,
Cation-exchange behavior of  Rf (T
Rf (T = 78 s) produced in the
= 78 s) produced in the  Cm(
Cm( O,5n) reaction was studied on a "one-atom-at-a-time" scale in 0.15-0.69 M H
O,5n) reaction was studied on a "one-atom-at-a-time" scale in 0.15-0.69 M H SO
SO /HNO
/HNO mixed solutions ([H
mixed solutions ([H ] = 1.0 M) using an automated ion-exchange separation apparatus coupled with a detection system for alpha-spectroscopy (AIDA). It was found that adsorption probabilities (%ads) of Rf on the cation-exchange resin decrease with increasing [HSO
] = 1.0 M) using an automated ion-exchange separation apparatus coupled with a detection system for alpha-spectroscopy (AIDA). It was found that adsorption probabilities (%ads) of Rf on the cation-exchange resin decrease with increasing [HSO
 ], showing successive formation of Rf sulfate complexes. Rf exhibits a weaker complex formation than the lighter homologues Zr and Hf.
], showing successive formation of Rf sulfate complexes. Rf exhibits a weaker complex formation than the lighter homologues Zr and Hf.
 SO
SO /HNO
/HNO mixed solution ([H
mixed solution ([H ] = 1.0 M)
] = 1.0 M)Li, Z.; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Kikuchi, Takahiro; Sch del, M.*; Nagame, Yuichiro; Liang, X. H.*; et al.
del, M.*; Nagame, Yuichiro; Liang, X. H.*; et al.
no journal, ,
Cation-exchange behavior of  Rf (T
Rf (T = 78 s) produced in the
= 78 s) produced in the  Cm(
Cm( O, 5n) reaction was studied on a "one-atom-at-a-time" scale in H
O, 5n) reaction was studied on a "one-atom-at-a-time" scale in H SO
SO (0.15-0.69 M)/HNO
(0.15-0.69 M)/HNO mixed solutions ([H
mixed solutions ([H ] = 1.0 M) using an automated ion-exchange separation apparatus coupled with the detection system for alpha-spectroscopy. It was found that adsorption probability (%ads) of
] = 1.0 M) using an automated ion-exchange separation apparatus coupled with the detection system for alpha-spectroscopy. It was found that adsorption probability (%ads) of  Rf on cation-exchange resin decreases with increasing [HSO
Rf on cation-exchange resin decreases with increasing [HSO
 ], showing a successive formation of its sulfate complexes. Rf exhibited much weaker formation of the complexes than the lighter homologues Zr and Hf, which is qualitatively in good agreement with theoretical calculations including relativistic effects.
], showing a successive formation of its sulfate complexes. Rf exhibited much weaker formation of the complexes than the lighter homologues Zr and Hf, which is qualitatively in good agreement with theoretical calculations including relativistic effects.
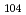 Rf with H
Rf with H SO
SO solutions
solutionsIkarashi, Satoshi*; Sueki, Keisuke*; Li, Z.*; Tsukada, Kazuaki; Nagame, Yuichiro
no journal, ,
To study ion-exchange kinetics on the diffusion processes of Rf and its homologues, Zr and Hf, with the cation-exchange resin in H SO
SO solutions, we obtained the fractional attainment of equilibrium of the carrier-free radiotracers
solutions, we obtained the fractional attainment of equilibrium of the carrier-free radiotracers  Zr and
Zr and 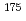 Hf adsorbed on the cation-exchange resin as a function of contact time within 300 seconds by the batch experiment. We discussed the dependence of functions of the fractional attainment of equilibrium on temperatures, concentrations of HSO
Hf adsorbed on the cation-exchange resin as a function of contact time within 300 seconds by the batch experiment. We discussed the dependence of functions of the fractional attainment of equilibrium on temperatures, concentrations of HSO - and radii of resins. It is result that the good agreement between experimental data and theoretical curves which the film diffusion control is the rate-determining step in the present ion-exchange process.
- and radii of resins. It is result that the good agreement between experimental data and theoretical curves which the film diffusion control is the rate-determining step in the present ion-exchange process.