Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Fe
Fe O
O grown on Al
grown on Al O
O ; Effects of postdeposition annealing studied by XMCD
; Effects of postdeposition annealing studied by XMCDNonaka, Yosuke*; Wakabayashi, Yuki*; Shibata, Goro; Sakamoto, Shoya*; Ikeda, Keisuke*; Chi, Z.*; Wan, Y.*; Suzuki, Masahiro*; Tanaka, Arata*; Tanaka, Masaaki*; et al.
Physical Review Materials (Internet), 7(4), p.044413_1 - 044413_10, 2023/04
Times Cited Count:5 Percentile:50.30(Materials Science, Multidisciplinary) TaS
TaS with perpendicular magnetic anisotropy; Comparison with Fe
with perpendicular magnetic anisotropy; Comparison with Fe TiS
TiS
Shibata, Goro; Won, C.*; Kim, J.*; Nonaka, Yosuke*; Ikeda, Keisuke*; Wan, Y.*; Suzuki, Masahiro*; Koide, Tsuneharu*; Tanaka, Arata*; Cheong, S.-W.*; et al.
Photon Factory Activity Report 2022 (Internet), 2 Pages, 2023/00
no abstracts in English
 Se
Se epitaxial thin films
epitaxial thin filmsNakano, Masaki*; Wang, Y.*; Yoshida, Satoshi*; Matsuoka, Hideki*; Majima, Yuki*; Ikeda, Keisuke*; Hirata, Yasuyuki*; Takeda, Yukiharu; Wadachi, Hiroki*; Kohama, Yoshimitsu*; et al.
Nano Letters, 19(12), p.8806 - 8810, 2019/12
Times Cited Count:61 Percentile:90.87(Chemistry, Multidisciplinary)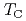 ferromagnetic semiconductor (Ga,Fe)Sb; X-ray magnetic circular dichroism and resonance photoemission spectroscopy studies
ferromagnetic semiconductor (Ga,Fe)Sb; X-ray magnetic circular dichroism and resonance photoemission spectroscopy studiesSakamoto, Shoya*; Tu, N. T.*; Takeda, Yukiharu; Fujimori, Shinichi; Hai, P. N.*; Anh, L. D.*; Wakabayashi, Yuki*; Shibata, Goro*; Horio, Masafumi*; Ikeda, Keisuke*; et al.
Physical Review B, 100(3), p.035204_1 - 035204_8, 2019/07
Times Cited Count:17 Percentile:59.00(Materials Science, Multidisciplinary)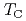 ferromagnetic semiconductor (Ga,Fe)Sb; X-ray magnetic circular dichroism and resonance photoemission spectroscopy studies
ferromagnetic semiconductor (Ga,Fe)Sb; X-ray magnetic circular dichroism and resonance photoemission spectroscopy studiesSakamoto, Shoya*; Tu, N. T.*; Takeda, Yukiharu; Fujimori, Shinichi; Hai, P. N.*; Anh, L. D.*; Wakabayashi, Yuki*; Shibata, Goro*; Horio, Masafumi*; Ikeda, Keisuke*; et al.
Physical Review B, 100(3), p.035204_1 - 035204_8, 2019/07
 VFeAsO
VFeAsO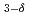 studied by X-ray magnetic circular dichroism
studied by X-ray magnetic circular dichroismHorio, Masafumi*; Takeda, Yukiharu; Namiki, Hiromasa*; Katagiri, Takao*; Wakabayashi, Yuki*; Sakamoto, Shoya*; Nonaka, Yosuke*; Shibata, Goro*; Ikeda, Keisuke*; Saito, Yuji; et al.
Journal of the Physical Society of Japan, 87(10), p.105001_1 - 105001_2, 2018/10
Times Cited Count:2 Percentile:19.05(Physics, Multidisciplinary) Co
Co )Fe
)Fe O
O (x=0-1) layers on Si(111) studied by soft X-ray magnetic circular dichroism
(x=0-1) layers on Si(111) studied by soft X-ray magnetic circular dichroismWakabayashi, Yuki*; Nonaka, Yosuke*; Takeda, Yukiharu; Sakamoto, Shoya*; Ikeda, Keisuke*; Chi, Z.*; Shibata, Goro*; Tanaka, Arata*; Saito, Yuji; Yamagami, Hiroshi; et al.
Physical Review Materials (Internet), 2(10), p.104416_1 - 104416_12, 2018/10
Times Cited Count:14 Percentile:43.11(Materials Science, Multidisciplinary)Torigoe, Shuhei*; Ishimoto, Yutaro*; Aoishi, Yuhei*; Murakawa, Hiroshi*; Matsumura, Daiju; Yoshii, Kenji; Yoneda, Yasuhiro; Nishihata, Yasuo; Kodama, Katsuaki; Tomiyasu, Keisuke*; et al.
Physical Review B, 93(8), p.085109_1 - 085109_5, 2016/02
Times Cited Count:6 Percentile:27.89(Materials Science, Multidisciplinary) O
O
Yoshii, Kenji; Mizumaki, Masaichiro*; Matsumoto, Keisuke*; Mori, Shigeo*; Endo, Naruki; Saito, Hiroyuki; Matsumura, Daiju; Kambe, Takashi*; Ikeda, Naoshi*
Journal of Physics; Conference Series, 428, p.012032_1 - 012032_5, 2013/04
Times Cited Count:15 Percentile:96.91(Chemistry, Applied)We have investigated a single crystal of multiferroic YbFe O
O . Its magnetic transition temperature was 250 K. The magnetization along the c-axis was much larger than that within the ab plane, indicating the Ising character of this system. The field-cooled magnetization became negative below 10 K. This was explained in terms of an antiparallel coupling between Yb and Fe moments. Magnetocaloric effects were also observed. The change of entropy was found to show a broad peak with a width of 100 K, which is favorable to application.
. Its magnetic transition temperature was 250 K. The magnetization along the c-axis was much larger than that within the ab plane, indicating the Ising character of this system. The field-cooled magnetization became negative below 10 K. This was explained in terms of an antiparallel coupling between Yb and Fe moments. Magnetocaloric effects were also observed. The change of entropy was found to show a broad peak with a width of 100 K, which is favorable to application.
Kobayashi, Masaki*; Niwa, Hideharu*; Harada, Yoshihisa*; Horiba, Koji*; Oshima, Masaharu*; Ofuchi, Hironori*; Terakura, Kiyoyuki*; Ikeda, Takashi; Koshigoe, Yuka*; Ozaki, Junichi*; et al.
Journal of Power Sources, 196(20), p.8346 - 8351, 2011/10
Times Cited Count:32 Percentile:65.60(Chemistry, Physical)The electronic structure of Co atoms in CoPc-based carbon catalysts, which were prepared by pyrolyzing a mixture of CoPc and phenol resin polymer up to 1000 C, has been investigated using XAFS analysis and HXPES. The Co K XAFS spectra show that most of the Co atoms are in the metallic state and small quantities of oxidized Co components are present in the samples even after acid washing to remove Co atoms. Based on the difference in probing depth between XAFS and HXPES, it was found that after acid washing, the surface region with the aggregated Co clusters is primarily composed of metallic Co. Since the electrochemical properties remain almost unchanged even after the acid washing process, the residual metallic and oxidized Co atoms themselves will hardly contribute to the ORR activity of the CoPc-based carbon cathode catalysts, implying that the active sites of the CoPc-based catalysts primarily consist of light elements such as C and N.
C, has been investigated using XAFS analysis and HXPES. The Co K XAFS spectra show that most of the Co atoms are in the metallic state and small quantities of oxidized Co components are present in the samples even after acid washing to remove Co atoms. Based on the difference in probing depth between XAFS and HXPES, it was found that after acid washing, the surface region with the aggregated Co clusters is primarily composed of metallic Co. Since the electrochemical properties remain almost unchanged even after the acid washing process, the residual metallic and oxidized Co atoms themselves will hardly contribute to the ORR activity of the CoPc-based carbon cathode catalysts, implying that the active sites of the CoPc-based catalysts primarily consist of light elements such as C and N.
 O
O
Matsumoto, Keisuke*; Koyama, Tsukasa*; Mori, Shigeo*; Yoshii, Kenji; Kambe, Takashi*; Ikeda, Naoshi*
IOP Conference Series; Materials Science and Engineering, 18(9), p.092047_1 - 092047_4, 2011/09
Times Cited Count:1 Percentile:50.51(Materials Science, Ceramics)Changes of charge ordered (CO) structure by partial substitution of Mn for Fe in YbFe O
O were investigated by a transmission electron microscope (TEM), incombination with conventional dielectric measurement. It is revealed that partial substitution of Mn
were investigated by a transmission electron microscope (TEM), incombination with conventional dielectric measurement. It is revealed that partial substitution of Mn for Fe
for Fe in YbFe
in YbFe O
O destroyed drastically the CO structure with the wave vector of q=
destroyed drastically the CO structure with the wave vector of q= 1/3 1/3 1/2
1/3 1/3 1/2 . Consequently polar clustering structure giving rise to honeycomb-shaped diffuse streaks was found in YbFeMnO
. Consequently polar clustering structure giving rise to honeycomb-shaped diffuse streaks was found in YbFeMnO . The random distribution of polar clustering structure gives rise to characteristic broad dielectric dispersion.
. The random distribution of polar clustering structure gives rise to characteristic broad dielectric dispersion.
 O
O
Matsumoto, Keisuke*; Koyama, Tsukasa*; Mori, Shigeo*; Yoshii, Kenji; Ikeda, Naoshi*
Journal of Physics; Conference Series, 320, p.012085_1 - 012085_5, 2011/09
Times Cited Count:0 Percentile:0.00(Physics, Condensed Matter)In order to clarify the stability of charge ordered structure (CO) in charge- and spinfrustrated ferrite YbFe O
O , we have investigated changes of the CO structure by partial substitution of Mn
, we have investigated changes of the CO structure by partial substitution of Mn for Fe
for Fe in YbFe
in YbFe O
O by a transmission electron microscope (TEM), incombination with conventional dielectric measurement. It is revealed that subtle substitution of Mn
by a transmission electron microscope (TEM), incombination with conventional dielectric measurement. It is revealed that subtle substitution of Mn for Fe
for Fe in YbFe
in YbFe O
O destroyed drastically the CO structure with the wave vector of q=
destroyed drastically the CO structure with the wave vector of q= 1/3 1/3 1/2
1/3 1/3 1/2 and, instead, induced polar clustering structure characterized by honeycomb shaped diffuse scatterings in the reciprocal space. The formation of polar clustering structure should be responsible for the characteristic dielectric dispersion.
and, instead, induced polar clustering structure characterized by honeycomb shaped diffuse scatterings in the reciprocal space. The formation of polar clustering structure should be responsible for the characteristic dielectric dispersion.
Niwa, Hideharu*; Kobayashi, Masaki*; Horiba, Koji*; Harada, Yoshihisa*; Oshima, Masaharu*; Terakura, Kiyoyuki*; Ikeda, Takashi; Koshigoe, Yuka*; Ozaki, Junichi*; Miyata, Seizo*; et al.
Journal of Power Sources, 196(3), p.1006 - 1011, 2011/02
Times Cited Count:93 Percentile:91.01(Chemistry, Physical)We report on the electronic structure of three different types of N-containing carbon-based cathode catalysts for polymer electrolyte fuel cells observed by hard X-ray photoemission spectroscopy. C 1s spectra show the importance of 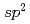 carbon network formation for the oxygen reduction reaction (ORR) activity. Samples having high oxygen reduction reaction activity in terms of oxygen reduction potential contain high concentration of graphite-like nitrogen. Based on a quantitative analysis of our results, the oxygen reduction reaction activity of the carbon-based cathode catalysts will be improved by increasing concentration of graphite-like nitrogen in a developed
carbon network formation for the oxygen reduction reaction (ORR) activity. Samples having high oxygen reduction reaction activity in terms of oxygen reduction potential contain high concentration of graphite-like nitrogen. Based on a quantitative analysis of our results, the oxygen reduction reaction activity of the carbon-based cathode catalysts will be improved by increasing concentration of graphite-like nitrogen in a developed 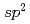 carbon network.
carbon network.
Fujimori, Shinichi; Terai, Kota; Takeda, Yukiharu; Okane, Tetsuo; Saito, Yuji; Kobayashi, Keisuke; Fujimori, Atsushi; Yamagami, Hiroshi*; Ikeda, Shugo; Matsuda, Tatsuma; et al.
Journal of the Physical Society of Japan, 75(Suppl.), p.99 - 101, 2006/08
Times Cited Count:0 Percentile:0.00(Physics, Multidisciplinary)In the present study, we have performed SX-ARPES experiments on some heavy Fermion (HF) uranium compounds such as UPd Al
Al using soft X-ray synchrotron radiation from BL23SU of SPring-8. UPd
using soft X-ray synchrotron radiation from BL23SU of SPring-8. UPd Al
Al is an HF uranium compound which has transitions into an antiferromagnetic phase at
is an HF uranium compound which has transitions into an antiferromagnetic phase at 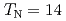 K and into a superconducting phase at
K and into a superconducting phase at 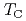 =2 K. To describe the various properties of this compound, the dual model for U 5
=2 K. To describe the various properties of this compound, the dual model for U 5 states is proposed. In this model, two of the three U 5f states are in a localized state while the third electron has delocalized character. We have obtained U 5f sensitive band structure of UPd
states is proposed. In this model, two of the three U 5f states are in a localized state while the third electron has delocalized character. We have obtained U 5f sensitive band structure of UPd Al
Al by SX-ARPES, and the results are compared with this scenario. Temperature dependence of the U 5
by SX-ARPES, and the results are compared with this scenario. Temperature dependence of the U 5 electronic structure in this compound is also discussed.
electronic structure in this compound is also discussed.
Tomiyasu, Keisuke*; Inami, Toshiya; Ikeda, Naoshi*
Physical Review B, 70(11), p.184411_1 - 184411_6, 2004/11
Times Cited Count:46 Percentile:83.83(Materials Science, Multidisciplinary)Below 
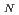 = 289 K, the antiferromagnet CoO exhibits magnetic long-range order described by a propagation vector along the trigonal axis, whereas the lattice is distorted monoclinicly along both the tetragonal and the trigonal axes. In order to elucidate this inconsistency, we performed neutron and synchrotron X-ray diffraction experiments on single crystals of CoO, and found a new series of magnetic reflections represented by a propagation vector along the tetragonal axis below
= 289 K, the antiferromagnet CoO exhibits magnetic long-range order described by a propagation vector along the trigonal axis, whereas the lattice is distorted monoclinicly along both the tetragonal and the trigonal axes. In order to elucidate this inconsistency, we performed neutron and synchrotron X-ray diffraction experiments on single crystals of CoO, and found a new series of magnetic reflections represented by a propagation vector along the tetragonal axis below 
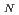 N. These results suggest an altered magnetic structure of CoO, which includes both the trigonal and the tetragonal propagation vectors, has a monoclinic symmetry and which is in accordance with the distorted lattice symmetry.
N. These results suggest an altered magnetic structure of CoO, which includes both the trigonal and the tetragonal propagation vectors, has a monoclinic symmetry and which is in accordance with the distorted lattice symmetry.
Fukushi, Keisuke*; Sasaki, Miwa*; Sato, Tsutomu*; Yanase, Nobuyuki; Amano, Hikaru; Ikeda, Hodaka*
Applied Geochemistry, 18(8), p.1267 - 1278, 2003/08
Times Cited Count:226 Percentile:95.61(Geochemistry & Geophysics)At Nishinomaki abandoned mine district, the water is acidic and contains much amounts of arsenic. However, arsenic concentration decreases downward without any artificial treatment. To understand the mechanism of the natural attenuation, the acid mine drainage and the ochreous precipitates were collected. The samples were analyzed by XRD, IR, ICP-MS and ion-chromatograph. The precipitates were investigated by selective extraction procedure. These results were interpreted with those calculated by the geochemical code. The contamination of water has been result from oxidation of pyrite and realgar and subsequent release of iron. The released ferrous iron transforms to ferric form by bacterial oxidation and then schwertmannite forms immediately. While the arsenic concentrations in the stream are lowered to background level at downstream, these in the ochreous precipitates are up to 60 mg/g. The iron hydroxide has been known to exhibit the high sorption affinity to arsenate. Hence, arsenic is effectively removed by the schwertmannite from the contaminated water and attenuated naturally.
Ikeda, Yoshitaka; Naito, Osamu; Ushigusa, Kenkichi; Sato, Masayasu; Kondoh, Takashi; Ide, Shunsuke; Seki, Masami; Nagashima, Keisuke; S.W.Wolfe*; Asakura, Nobuyuki; et al.
Nuclear Fusion, 34(6), p.871 - 880, 1994/00
Times Cited Count:15 Percentile:49.91(Physics, Fluids & Plasmas)no abstracts in English
Imai, Tsuyoshi; Ushigusa, Kenkichi; Ikeda, Yoshitaka; Naito, Osamu; Yoshida, Hidetoshi; Seki, Masami; Itami, Kiyoshi; Nagashima, Keisuke; Uehara, Kazuya; Nagashima, Takashi
Kaku Yugo Kenkyu, 65(SPECIAL ISSUE), p.99 - 118, 1991/03
no abstracts in English
Tsuji, Shunji; Ushigusa, Kenkichi; Ikeda, Yoshitaka; Imai, Tsuyoshi; Itami, Kiyoshi; Nemoto, Masahiro; Nagashima, Keisuke; Koide, Yoshihiko; Kawano, Yasunori; Fukuda, Takeshi; et al.
Physical Review Letters, 64(9), p.1023 - 1026, 1990/02
Times Cited Count:56 Percentile:88.32(Physics, Multidisciplinary)no abstracts in English
Ushigusa, Kenkichi; Imai, Tsuyoshi; Ikeda, Yoshitaka; Sakamoto, Keishi; F.X.Soeldner*; ; Tsuji, Shunji; Shimizu, Katsuhiro; Naito, Osamu; Uehara, Kazuya; et al.
Nuclear Fusion, 29(2), p.265 - 276, 1989/02
Times Cited Count:17 Percentile:58.16(Physics, Fluids & Plasmas)no abstracts in English