Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Katsumura, Kosuke*; Takagi, Junichi*; Hosomi, Kenji*; Miyahara, Naoya*; Koma, Yoshikazu; Imoto, Jumpei; Karasawa, Hidetoshi; Miwa, Shuhei; Shiotsu, Hiroyuki; Hidaka, Akihide*; et al.
Nihon Genshiryoku Gakkai-Shi ATOMO , 65(11), p.674 - 679, 2023/11
, 65(11), p.674 - 679, 2023/11
no abstracts in English
Miwa, Shuhei; Karasawa, Hidetoshi; Nakajima, Kunihisa; Kino, Chiaki*; Suzuki, Eriko; Imoto, Jumpei
JAEA-Data/Code 2021-022, 32 Pages, 2023/01
The improved model for cesium (Cs) chemisorption onto stainless steel (SS) in the fission product (FP) chemistry database named ECUME was incorporated into the severe accident (SA) analysis code SAMPSON for the more accurate estimation of Cs distribution within nuclear reactor vessels in the TEPCO's Fukushima Daiichi Nuclear Power Station (1F). The SAMPSON with the improved model was verified based on the analysis results reproducing the experimental results which were subjected to the modeling of Cs chemisorption behavior. Then, the experiment in the facility with the temperature gradient tube to simulate SA conditions such as temperature decrease and aerosol formation was analyzed to confirm availability of the improved model to the analysis of Cs chemisorption onto SS. The SAMPSON with the improved model successfully reproduced the experimental results, which indicates that the improved model and the analytical method such as setting a method of node-junction, models of aerosol formation and the calculation method of saturated CsOH vapor pressure can be applicable to the analysis of Cs chemisorption behavior. As the information on water-solubility of Cs deposits was also prerequisite to estimate the Cs distribution in the 1F because Cs can be transported through aqueous phase after the SA, the water-solubility of chemisorbed Cs compounds was investigated. The chemisorbed compounds on SS304 have been identified to CsFeO at 873 K to 973 K with higher water-solubility, CsFeSiO
at 873 K to 973 K with higher water-solubility, CsFeSiO at 973 K to 1273 K and Cs
at 973 K to 1273 K and Cs Si
Si O
O at 1073 K to 1273 K with lower water-solubility. From these results, the water-solubility of chemisorbed Cs compounds can be estimated according to the SA analysis conditions such as temperature in the reactor and the CsOH concentration affecting the amount of chemisorbed Cs.
at 1073 K to 1273 K with lower water-solubility. From these results, the water-solubility of chemisorbed Cs compounds can be estimated according to the SA analysis conditions such as temperature in the reactor and the CsOH concentration affecting the amount of chemisorbed Cs.
Rizaal, M.; Miwa, Shuhei; Suzuki, Eriko; Imoto, Jumpei; Osaka, Masahiko; Gou llo, M.*
llo, M.*
ACS Omega (Internet), 6(48), p.32695 - 32708, 2021/12
Times Cited Count:5 Percentile:25.61(Chemistry, Multidisciplinary)Imoto, Jumpei; Nakajima, Kunihisa; Osaka, Masahiko
Nihon Genshiryoku Gakkai Wabun Rombunshi, 20(4), p.179 - 187, 2021/12
Some of the Cs inside the Fukushima Daiichi Nuclear Power Station would be deposited in chemical forms such as CsI and Cs MoO
MoO . Since Cs compounds are generally water-soluble, it is predicted that the migration of Cs through the aqueous phase occurs in the long term. Knowledge of the solubility in water is required as basic data for such migration behavior evaluation. Therefore, this study was conducted to investigate the dissolution properties of CsI and Cs
. Since Cs compounds are generally water-soluble, it is predicted that the migration of Cs through the aqueous phase occurs in the long term. Knowledge of the solubility in water is required as basic data for such migration behavior evaluation. Therefore, this study was conducted to investigate the dissolution properties of CsI and Cs MoO
MoO in water at 20
in water at 20 C and 25
C and 25 C. The solubilities of CsI at 25
C. The solubilities of CsI at 25 C calculated using thermodynamic data and the Pitzer ion interaction model were in good agreement with the literature value. It was found that the literature value of CsI at around room temperature is highly reliable. The experimental value of CsI at 20
C calculated using thermodynamic data and the Pitzer ion interaction model were in good agreement with the literature value. It was found that the literature value of CsI at around room temperature is highly reliable. The experimental value of CsI at 20 C obtained by the OECD test guideline 105 flask method (test guideline) was also in good agreement with the literature value. The measured solubility of Cs
C obtained by the OECD test guideline 105 flask method (test guideline) was also in good agreement with the literature value. The measured solubility of Cs MoO
MoO was 256.8
was 256.8  6.2 (g/100 g H
6.2 (g/100 g H O) at 20
O) at 20 C using the test guideline. This measured solubility of Cs
C using the test guideline. This measured solubility of Cs MoO
MoO was found to be comparable to those of other alkaline molybdates and considered to be more reliable than the literature value.
was found to be comparable to those of other alkaline molybdates and considered to be more reliable than the literature value.
Miwa, Shuhei; Miyahara, Naoya*; Nakajima, Kunihisa; Imoto, Jumpei; Suzuki, Eriko
Nihon Genshiryoku Gakkai-Shi ATOMO , 63(12), p.825 - 829, 2021/12
, 63(12), p.825 - 829, 2021/12
In the BWR severe accident, it was indicated that the control material boron significantly influences chemical behavior and transition behavior of cesium, which is important from the viewpoint of exposure, and it causes great uncertainty in the prediction of environmental release and distribution in the reactor. Therefore, in order to elucidate the important chemistry to be considered in severe accident analysis, we have developed the experimental setup that enables the evaluation of chemistry during transportation in the reactor, and evaluated cesium chemistry. Based on the results, we developed the chemistry database named ECUME composed of datasets and models that are the basis of chemical reaction analysis so that chemical behavior could be evaluated by severe accident analysis code.
Miyahara, Naoya; Miwa, Shuhei; Gou llo, M.*; Imoto, Jumpei; Horiguchi, Naoki; Sato, Isamu*; Osaka, Masahiko
llo, M.*; Imoto, Jumpei; Horiguchi, Naoki; Sato, Isamu*; Osaka, Masahiko
Journal of Nuclear Science and Technology, 57(12), p.1287 - 1296, 2020/12
Times Cited Count:9 Percentile:65.08(Nuclear Science & Technology)In order to clarify the cesium iodide (CsI) transport behavior with a focus on the mechanisms of gaseous iodine formation in the reactor coolant system of LWR under a severe accident condition, a reproductive experiment of CsI transport behavior was conducted using a facility equipped with a thermal gradient tube. Various analyses on deposits and airborne materials during transportation could elucidate two mechanisms for the gaseous iodine formation. One was the gaseous phase chemical reaction in Cs-I-O-H system at relatively high-temperature region, which led to gaseous iodine transport to the lower temperature region without any further changes in gas species due to the kinetics limitation effects. The other one was the chemical reactions related to condensed phase of CsI, namely those of CsI deposits on walls with surface of stainless steel to form Cs CrO
CrO compound and CsI aerosol particles with steam, which were newly found in this study.
compound and CsI aerosol particles with steam, which were newly found in this study.
Miwa, Shuhei; Nakajima, Kunihisa; Miyahara, Naoya; Nishioka, Shunichiro; Suzuki, Eriko; Horiguchi, Naoki; Liu, J.; Miradji, F.; Imoto, Jumpei; Afiqa, B. M.; et al.
Mechanical Engineering Journal (Internet), 7(3), p.19-00537_1 - 19-00537_11, 2020/06
We constructed the fission product (FP) chemistry database named ECUME for LWR severe accident. This version of ECUME is equipped with dataset of the chemical reactions and their kinetics constants for the reactions of cesium(Cs)-iodine(I)-boron(B)-molybdenum(Mo)-oxygen(O)-hydrogen(H) system in gas phase, the elemental model for the high temperature chemical reaction of Cs with stainless steel applied as the structural material in a reactor, and thermodynamic data for CsBO vapor species and solids of Cs
vapor species and solids of Cs Si
Si O
O and CsFeSiO
and CsFeSiO for these chemical reactions. The ECUME will provide estimation of Cs distribution due to the evaluation of effects of interaction with BWR control material B and stainless steel on Cs behavior in the Fukushima Daiichi Nuclear Power Station.
for these chemical reactions. The ECUME will provide estimation of Cs distribution due to the evaluation of effects of interaction with BWR control material B and stainless steel on Cs behavior in the Fukushima Daiichi Nuclear Power Station.
Miwa, Shuhei; Takase, Gaku; Imoto, Jumpei; Nishioka, Shunichiro; Miyahara, Naoya; Osaka, Masahiko
Journal of Nuclear Science and Technology, 57(3), p.291 - 300, 2020/03
Times Cited Count:10 Percentile:66.83(Nuclear Science & Technology)For the evaluation of transport behavior of control material boron in a severe accident of BWR from the viewpoint of chemical effects on cesium and iodine behavior, boron chemistry during transportation in the high temperature region above 400 K was experimentally investigated. The heating tests of boron oxide specimen were conducted using the dedicated experimental apparatus reproducing fission product release and transport in steam atmosphere. Released boron oxide vapor was deposited above 1,000 K by the condensation onto stainless steel. The boron deposits and/or vapors significantly reacted with stainless steel above 1,000 K and formed the stable iron-boron mixed oxide (FeO) BO
BO . These results indicate that released boron from degraded BWR control blade in a severe accident could remain in the high temperature region such as a Reactor Pressure Vessel. Based on these results, it can be said that the existence of boron deposits in the high temperature region would decrease the amount of transported cesium vapors from a Reactor Pressure Vessel due to possible formation of low volatile cesium borate compounds by the reaction of boron deposits with cesium vapors.
. These results indicate that released boron from degraded BWR control blade in a severe accident could remain in the high temperature region such as a Reactor Pressure Vessel. Based on these results, it can be said that the existence of boron deposits in the high temperature region would decrease the amount of transported cesium vapors from a Reactor Pressure Vessel due to possible formation of low volatile cesium borate compounds by the reaction of boron deposits with cesium vapors.
Miwa, Shuhei; Miyahara, Naoya; Nakajima, Kunihisa; Nishioka, Shunichiro; Suzuki, Eriko; Horiguchi, Naoki; Liu, J.; Miradji, F.; Imoto, Jumpei; Afiqa, B. M.; et al.
Proceedings of 27th International Conference on Nuclear Engineering (ICONE-27) (Internet), 8 Pages, 2019/05
We constructed the first version of fission product (FP) chemistry database named ECUME for LWR severe accident. The first version of ECUME is equipped with dataset of the chemical reactions and their kinetics constants for the reactions of cesium(Cs)-iodine(I)-boron(B)-molybdenum(Mo)-oxygen(O)-hydrogen(H) system in gas phase, the elemental model for the high temperature chemical reaction of Cs with stainless steel, and thermodynamic data for CsBO vapor species and solids of Cs
vapor species and solids of Cs Si
Si O
O and CsFeSiO
and CsFeSiO . The ECUME will provide more accurate estimation of Cs distribution due to the evaluation of effects of interaction with BWR control material B and stainless steel on Cs behavior in the Fukushima Daiichi Nuclear Power Station.
. The ECUME will provide more accurate estimation of Cs distribution due to the evaluation of effects of interaction with BWR control material B and stainless steel on Cs behavior in the Fukushima Daiichi Nuclear Power Station.
Imoto, Jumpei; Miwa, Shuhei; Osaka, Masahiko
Proceedings of International Topical Workshop on Fukushima Decommissioning Research (FDR 2019) (Internet), 4 Pages, 2019/05
Boron (B) oxidative vaporization processes from the representative alloys of Fe-B and Zr-B formed in the mixed melt of BWR control material boron carbide, stainless steel and Zircaloy were experimentally investigated toward the construction of B release model under severe accident. The results show that B oxidative vaporization from ZrB would proceed in the formation of ZrO
would proceed in the formation of ZrO and B
and B O
O due to the oxidation of ZrB
due to the oxidation of ZrB , followed by the formation of volatile H-B-O vapor species by the reaction of B
, followed by the formation of volatile H-B-O vapor species by the reaction of B O
O with steam. On the other hand, for Fe
with steam. On the other hand, for Fe B and FeB, the B oxidative vaporization processes would proceed in the same manner. Complex Fe-B-O compounds formation in addition to amorphous B
B and FeB, the B oxidative vaporization processes would proceed in the same manner. Complex Fe-B-O compounds formation in addition to amorphous B O
O were observed by the oxidation of Fe
were observed by the oxidation of Fe B and FeB. Then the B vaporization would occur by the formation of volatile H-B-O compound by the reaction of B
B and FeB. Then the B vaporization would occur by the formation of volatile H-B-O compound by the reaction of B O
O , which were derived from both oxidation of Fe
, which were derived from both oxidation of Fe B and decomposition of Fe-B-O compounds.
B and decomposition of Fe-B-O compounds.
Tanaka, Taiki*; Narikiyo, Yoshihiro*; Morita, Kosuke*; Fujita, Kunihiro*; Kaji, Daiya*; Morimoto, Koji*; Yamaki, Sayaka*; Wakabayashi, Yasuo*; Tanaka, Kengo*; Takeyama, Mirei*; et al.
Journal of the Physical Society of Japan, 87(1), p.014201_1 - 014201_9, 2018/01
Times Cited Count:23 Percentile:75.18(Physics, Multidisciplinary)Excitation functions of quasielastic scattering cross sections for the  Ca +
Ca +  Pb,
Pb,  Ti +
Ti +  Pb, and
Pb, and  Ca +
Ca +  Cm reactions were successfully measured by using the gas-filled recoil-ion separator GARIS. Fusion barrier distributions were extracted from these data, and compared with the coupled-channels calculations. It was found that the peak energies of the barrier distributions for the
Cm reactions were successfully measured by using the gas-filled recoil-ion separator GARIS. Fusion barrier distributions were extracted from these data, and compared with the coupled-channels calculations. It was found that the peak energies of the barrier distributions for the  Ca +
Ca +  Pb and
Pb and  Ti +
Ti +  Pb systems coincide with those of the 2n evaporation channel cross sections for the systems, while that of the
Pb systems coincide with those of the 2n evaporation channel cross sections for the systems, while that of the  Ca +
Ca +  Cm is located slightly below the 4n evaporation ones. This results provide us helpful information to predict the optimum beam energy to synthesize superheavy nuclei.
Cm is located slightly below the 4n evaporation ones. This results provide us helpful information to predict the optimum beam energy to synthesize superheavy nuclei.
Eichler, R.*; Asai, Masato; Brand, H.*; Chiera, N. M.*; Di Nitto, A.*; Dressler, R.*; D llmann, Ch. E.*; Even, J.*; Fangli, F.*; Goetz, M.*; et al.
llmann, Ch. E.*; Even, J.*; Fangli, F.*; Goetz, M.*; et al.
EPJ Web of Conferences, 131, p.07005_1 - 07005_7, 2016/12
Times Cited Count:3 Percentile:71.33(Chemistry, Inorganic & Nuclear)In recent years gas-phase chemical studies assisted by physical pre-separation allowed for the productions and investigations of fragile single molecular species of superheavy elements. The latest highlight is the formation of very volatile hexacarbonyl compound of element 106, Sg(CO) . Following this success, second-generation experiments were performed to measure the first bond dissociation energy between the central metal atom and the surrounding ligand. The method using a tubular decomposition reactor was developed and successfully applied to short-lived Mo(CO)
. Following this success, second-generation experiments were performed to measure the first bond dissociation energy between the central metal atom and the surrounding ligand. The method using a tubular decomposition reactor was developed and successfully applied to short-lived Mo(CO) , W(CO)
, W(CO) , and Sg(CO)
, and Sg(CO) .
.
Yamasaki, Shinya*; Imoto, Jumpei*; Furuki, Genki*; Ochiai, Asumi*; Onuki, Toshihiko; Sueki, Keisuke*; Namba, Kenji*; Ewing, R. C.*; Utsunomiya, Satoshi*
Science of the Total Environment, 551-552, p.155 - 162, 2016/05
Times Cited Count:36 Percentile:70.81(Environmental Sciences)Cesium-137 ( Cs) of estuary sediment impacted by the FDNPP was measured. Increasing radioactivity was observed from surface to bottom. 90% of the
Cs) of estuary sediment impacted by the FDNPP was measured. Increasing radioactivity was observed from surface to bottom. 90% of the  Cs was strongly bound to clay minerals in the estuary sediments. These results suggest that
Cs was strongly bound to clay minerals in the estuary sediments. These results suggest that  Cs is being transported from contaminated paddy fields to the estuary.
Cs is being transported from contaminated paddy fields to the estuary.
 and W(CO)
and W(CO)
Usoltsev, I.*; Eichler, R.*; Wang, Y.*; Even, J.*; Yakushev, A.*; Haba, Hiromitsu*; Asai, Masato; Brand, H.*; Di Nitto, A.*; D llmann, Ch. E.*; et al.
llmann, Ch. E.*; et al.
Radiochimica Acta, 104(3), p.141 - 151, 2016/03
Times Cited Count:34 Percentile:94.43(Chemistry, Inorganic & Nuclear)Conditions of the production and decomposition of hexacarbonyl complexes of short-lived Mo and W isotopes were investigated to study thermal stability of the heaviest group 6 hexacarbonyl complex Sg(CO) . A tubular flow reactor was tested to decompose the hexacarbonyl complexes and to extract the first bond dissociation energies. A silver was found to be the most appropriate reaction surface to study the decomposition of the group 6 hexacarbonyl. It was found that the surface temperature at which the decomposition occurred was correlated to the first bond dissociation energy of Mo(CO)
. A tubular flow reactor was tested to decompose the hexacarbonyl complexes and to extract the first bond dissociation energies. A silver was found to be the most appropriate reaction surface to study the decomposition of the group 6 hexacarbonyl. It was found that the surface temperature at which the decomposition occurred was correlated to the first bond dissociation energy of Mo(CO) and W(CO)
and W(CO) , indicating that the first bond dissociation energy of Sg(CO)
, indicating that the first bond dissociation energy of Sg(CO) could be determined with this technique.
could be determined with this technique.
Kaneko, Makoto*; Iwata, Hajime; Shiotsu, Hiroyuki; Masaki, Shota*; Kawamoto, Yuji*; Yamasaki, Shinya*; Nakamatsu, Yuki*; Imoto, Jumpei*; Furuki, Genki*; Ochiai, Asumi*; et al.
Frontiers in Energy Research (Internet), 3, p.37_1 - 37_10, 2015/09
The mobility of the aggregates of submicron-sized sheet aluminosilicate in the surface environment is a key factor controlling the current Cs migration in Fukushima.
Kaji, Daiya*; Morimoto, Koji*; Wakabayashi, Yasuo*; Takeyama, Mirei*; Yamaki, Sayaka*; Tanaka, Kengo*; Haba, Hiromitsu*; Huang, M.*; Murakami, Masashi*; Kanaya, Jumpei*; et al.
JPS Conference Proceedings (Internet), 6, p.030107_1 - 030107_4, 2015/06
Performance of the new gas-filled recoil ion separator GARIS-II was investigated using asymmetric  Ne-induced fusion reactions. The use of He-H
Ne-induced fusion reactions. The use of He-H mixture gas for the gas-filled magnet significantly reduced background scattered particles detected at the focal-plane Si detector, and increased a transmission of the asymmetric reaction products. A target-identification system was newly installed for efficient measurements of excitation functions without changing beam energy nor target.
mixture gas for the gas-filled magnet significantly reduced background scattered particles detected at the focal-plane Si detector, and increased a transmission of the asymmetric reaction products. A target-identification system was newly installed for efficient measurements of excitation functions without changing beam energy nor target.
 Nb and
Nb and 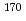 Ta for chemical studies of element 105, Db, using the GARIS gas-jet system
Ta for chemical studies of element 105, Db, using the GARIS gas-jet systemHuang, M.*; Haba, Hiromitsu*; Murakami, Masashi*; Asai, Masato; Kaji, Daiya*; Kanaya, Jumpei*; Kasamatsu, Yoshitaka*; Kikunaga, Hidetoshi*; Kikutani, Yuki*; Komori, Yukiko*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 304(2), p.845 - 849, 2015/05
Times Cited Count:3 Percentile:23.54(Chemistry, Analytical)A technique to utilize radioisotopes of Nb and Ta was developed for chemical studies of element 105, Db, by coupling a gas-jet transport system to the RIKEN gas-filled recoil ion separator (GARIS). The short-lived  Nb and
Nb and 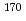 Ta were produced with nuclear reactions using a
Ta were produced with nuclear reactions using a  F beam whose energy was the same as that to produce
F beam whose energy was the same as that to produce  Db. Then, they were separated with GARIS and extracted to a chemistry laboratory with the gas-jet transport system. By changing only magnetic field of GARIS and inserting an energy degrader and a shutter for recoil ions, we could deliver the
Db. Then, they were separated with GARIS and extracted to a chemistry laboratory with the gas-jet transport system. By changing only magnetic field of GARIS and inserting an energy degrader and a shutter for recoil ions, we could deliver the  Nb and
Nb and 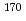 Ta to a chemistry device for
Ta to a chemistry device for  Db without changing other experimental conditions.
Db without changing other experimental conditions.
Even, J.*; Ackermann, D.*; Asai, Masato; Block, M.*; Brand, H.*; Di Nitto, A.*; D llmann, Ch. E.*; Eichler, R.*; Fan, F.*; Haba, Hiromitsu*; et al.
llmann, Ch. E.*; Eichler, R.*; Fan, F.*; Haba, Hiromitsu*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(3), p.2457 - 2466, 2015/03
Times Cited Count:15 Percentile:74.22(Chemistry, Analytical)Rapid In situ synthesis of metal carbonyl complexes has been demonstrated using short-lived isotopes produced in nuclear fission and fusion reactions. The short-lived isotopes with high recoil energy directly react with carbon-monoxides and form carbonyl complexes. Only highly volatile complexes were fast transported in a gas stream to counting and chemistry devices. Short-lived Mo, Tc, Ru, Rh, W, Re, Os, and Ir were found to form volatile carbonyl complexes, while no volataile complex of Hf and Ta were detected. This technique has been applied to a chemical investigation of the superheavy element Sg (atomic number 106), and will be applicable to various fields of nuclear science with short-lived transition metal isotopes.
Even, J.*; Yakushev, A.*; D llmann, Ch. E.*; Haba, Hiromitsu*; Asai, Masato; Sato, Tetsuya; Brand, H.*; Di Nitto, A.*; Eichler, R.*; Fan, F. L.*; et al.
llmann, Ch. E.*; Haba, Hiromitsu*; Asai, Masato; Sato, Tetsuya; Brand, H.*; Di Nitto, A.*; Eichler, R.*; Fan, F. L.*; et al.
Science, 345(6203), p.1491 - 1493, 2014/09
Times Cited Count:67 Percentile:83.06(Multidisciplinary Sciences)A new superheavy element complex, a seaborgium carbonyl, has been successfully synthesized, and its adsorption property has been studied using a cryo-thermochromatography and  -detection apparatus COMPACT. Nuclear reaction products of short-lived
-detection apparatus COMPACT. Nuclear reaction products of short-lived  Sg preseparated with a gas-filled recoil ion separator GARIS at RIKEN were directly injected into a gas cell filled with He/CO mixture gas, and chemical reaction products of volatile carbonyl complexes were trasported to COMPACT. The Sg carbonyl complex detected with COMPACT was found to be very volatile with adsorption enthalpy of
Sg preseparated with a gas-filled recoil ion separator GARIS at RIKEN were directly injected into a gas cell filled with He/CO mixture gas, and chemical reaction products of volatile carbonyl complexes were trasported to COMPACT. The Sg carbonyl complex detected with COMPACT was found to be very volatile with adsorption enthalpy of 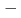 50 kJ/mol, from which we have concluded that this complex should be a Sg hexacarbonyl Sg(CO)
50 kJ/mol, from which we have concluded that this complex should be a Sg hexacarbonyl Sg(CO) . This is the first synthesis of organometallic compounds of transactinide elements for which only simple inorganic comounds have been synthesized so far.
. This is the first synthesis of organometallic compounds of transactinide elements for which only simple inorganic comounds have been synthesized so far.
 Db in the
Db in the  Cm(
Cm( F,5
F,5 )
) Db reaction and decay properties of
Db reaction and decay properties of  Db and
Db and 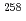 Lr
LrHaba, Hiromitsu*; Huang, M.*; Kaji, Daiya*; Kanaya, Jumpei*; Kudo, Yuki*; Morimoto, Koji*; Morita, Kosuke*; Murakami, Masashi*; Ozeki, Kazutaka*; Sakai, Ryutaro*; et al.
Physical Review C, 89(2), p.024618_1 - 024618_11, 2014/02
Times Cited Count:27 Percentile:81.97(Physics, Nuclear)