Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
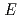 h analysis (Contract research)
h analysis (Contract research)Otsuka, Ichiro; Taki, Hiroshi*; Yamaguchi, Tetsuji; Iida, Yoshihisa; Yamada, Fumika; Inada, Daisuke*; Tanaka, Tadao
JAEA-Research 2008-043, 101 Pages, 2008/03
The influence of carbon steel overpack corrosion on redox potential (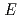 h) of bentonite pore water under geological disposal environment was investigated. The thermodynamics data of corrosion products, the corrosion rate of carbon steel, and the information on cathode reactions were acquired by experiments and literature survey. We conducted preliminary analysis of
h) of bentonite pore water under geological disposal environment was investigated. The thermodynamics data of corrosion products, the corrosion rate of carbon steel, and the information on cathode reactions were acquired by experiments and literature survey. We conducted preliminary analysis of 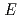 h, ascertained the validity of Phreeq C and identified important points on the analysis. Results were summarized as follows. (1) Thermodynamic data of Fe
h, ascertained the validity of Phreeq C and identified important points on the analysis. Results were summarized as follows. (1) Thermodynamic data of Fe , FeOH
, FeOH , Fe(OH)
, Fe(OH) (aq), Fe(OH)
(aq), Fe(OH)
 , Fe(OH)
, Fe(OH)
 , Fe
, Fe , FeS
, FeS (pyrite), FeCO
(pyrite), FeCO (siderite),Fe(OH)
(siderite),Fe(OH) (s), Fe
(s), Fe O
O (magnetite), Fe(cr) were determined by literature survey. The solubility product of Fe
(magnetite), Fe(cr) were determined by literature survey. The solubility product of Fe CO
CO (OH)
(OH) was determined experimentally, and thermodynamic data were estimated. (2) The corrosion rate of carbon steel was obtained as a function of pH and sulfide ion concentration. (3) After corrosion tests of carbon steel, no CH
was determined experimentally, and thermodynamic data were estimated. (2) The corrosion rate of carbon steel was obtained as a function of pH and sulfide ion concentration. (3) After corrosion tests of carbon steel, no CH , HS
, HS and H
and H S, the reduction product of CO
S, the reduction product of CO
 and SO
and SO
 ,were not detected in liquid and gas phases. (4) Preliminary analysis showed that the redox couple changed as HS
,were not detected in liquid and gas phases. (4) Preliminary analysis showed that the redox couple changed as HS (aq)/SO
(aq)/SO
 , CH
, CH (aq)/CO
(aq)/CO
 , H
, H (aq)/H
(aq)/H O during the evaluation period. After 1000 years,
O during the evaluation period. After 1000 years, 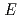 h attained about -500 to -600 mV (vs. NHE) or -750 mV controlled by CH
h attained about -500 to -600 mV (vs. NHE) or -750 mV controlled by CH (aq)/CO
(aq)/CO
 ,or H
,or H (aq)/H
(aq)/H O, respectively.
O, respectively.
Mukai, Masayuki; Ueda, Masato; Inada, Daisuke; Yukawa, Kazuhiko; Maeda, Toshikatsu; Iida, Yoshihisa
Proceedings of International Symposium NUCEF 2005, p.219 - 224, 2005/08
For better quantitative understanding of radionuclide migration for safety assessment of geologic disposal, JAERI has been conducting experimental and modeling studies on influences of humic substances, highly alkaline conditions and colloids on sorptive and diffusional behavior of TRU in geologic materials. In the absence of fulvic acid, one of humic substances, diffusion of Am through a tuff sample was not detected. By adding fulvic acid, Am was detected in the downstream cell, which indicates the diffusion through the sample. Highly alkaline conditions arisen from cementitious materials may spread by altering chemical and physical properties of geologic materials. Through-diffusion experiments of alkaline species in granite showed that the effective diffusion coefficient of Ca and OH
and OH in a cement-equilibrated aqueous solution were found to be higher by almost two orders of magnitude than Na
in a cement-equilibrated aqueous solution were found to be higher by almost two orders of magnitude than Na and OH
and OH in a NaOH solution. Radionuclide migration can be enhanced by colloids, and thus a calculation code describing the effect of colloids on radionuclide migration has been required.
in a NaOH solution. Radionuclide migration can be enhanced by colloids, and thus a calculation code describing the effect of colloids on radionuclide migration has been required.
Iida, Yoshihisa; Yamaguchi, Tetsuji; Inada, Daisuke; Tanaka, Tadao; Otsuka, Ichiro*
no journal, ,
no abstracts in English
Otsuka, Ichiro*; Tanaka, Tadao; Inada, Daisuke*
no journal, ,
The experiments to elucidate physico-chemical behavior of iron in the low oxygen and reducing condition of deep underground geology have been performed carefully in the glove box controlling oxygen concentration. It is necessary to protect oxidation by oxygen for XRD measurement of Fe(II) compounds that easily oxidize in atmosphere. In the present research, the method to measure XRD of the Fe(II) compounds was proposed. In the argon gas circulating type glove box controlled in oxygen concentration less than 1 ppm, Fe(OH) was prepared. Resulting Fe(OH)
was prepared. Resulting Fe(OH) was pasted to surface of glass cell, and was sealed by Kapton film. The glass cell was taken out into atmosphere from a glove box and XRD measurement was performed. As to the cell sealed up by Kapton film, only peaks of Fe(OH)
was pasted to surface of glass cell, and was sealed by Kapton film. The glass cell was taken out into atmosphere from a glove box and XRD measurement was performed. As to the cell sealed up by Kapton film, only peaks of Fe(OH) was observed. This experimental result shows that the XRD measurement of Fe(OH)
was observed. This experimental result shows that the XRD measurement of Fe(OH) in atmosphere is possible by sealing up the sample on glass cell by Kapton film.
in atmosphere is possible by sealing up the sample on glass cell by Kapton film.
Sakamaki, Keiko; Otsuka, Ichiro*; Iida, Yoshihisa; Inada, Daisuke*; Kamoshida, Michio; Kataoka, Masaharu; Yamaguchi, Tetsuji; Tanaka, Tadao
no journal, ,
no abstracts in English