Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Irisawa, Eriko; Kato, Chiaki
Journal of Nuclear Materials, 591, p.154914_1 - 154914_10, 2024/04
Times Cited Count:0The amount of corrosion of austenitic stainless-steel R-SUS304ULC was evaluated considering the changes in solution composition and boiling during actual concentration operations. Austenitic stainless-steel R-SUS304ULC is the structural material of the highly radioactive liquid waste concentrator in Japanese spent fuel reprocessing plant, which treats highly corrosive nitric acid solutions during enrichment operations. The study results show that it is necessary to focus on nitric acid concentrations, oxidizing metal ion concentrations, and decompression boiling as factors that accelerate the corrosion rate of stainless steel because of cathodic reaction activation.
Yamashita, Naoki; Irisawa, Eriko; Kato, Chiaki; Sano, Naruto; Tagami, Susumu
JAEA-Technology 2022-035, 29 Pages, 2023/03
In the treatment process of the current commercial reprocessing plant (Rokkasho Reprocessing Plant), the high-level liquid waste concentrator is the equipment that treats the most corrosive solution. In the high-level liquid waste concentrator, the extracted liquid waste after separation of uranium and plutonium is heated, concentrated, and reduced in volume. Therefore, the amount of gamma- rays emitted from fission products and the concentration of corrosive metal ion species such as neptunium-237 ( Np) are the highest in the reprocessing process, and the amount of corrosion in the high-level liquid waste concentrate canner is expected to be large. In this study, in order to clarify the effect of gamma-rays on the corrosion reaction of stainless steel in nitric acid solutions containing
Np) are the highest in the reprocessing process, and the amount of corrosion in the high-level liquid waste concentrate canner is expected to be large. In this study, in order to clarify the effect of gamma-rays on the corrosion reaction of stainless steel in nitric acid solutions containing  Np from the electrochemical viewpoint, the corrosion test apparatus for heat transfer surfaces in an airtight concrete cell at the Waste Safety TEsting Facility (WASTEF) of Nuclear Science Research Institute was modified to enable electrochemical measurements under gamma-ray irradiation. The effect of gamma-rays on the corrosion reaction taking place on the stainless steel surface was discussed from the electrochemical test results obtained. As a result, changes in the immersion potentials of stainless steel and the polarization curves due to chemical species caused by radiolysis of gamma-ray irradiation were confirmed.
Np from the electrochemical viewpoint, the corrosion test apparatus for heat transfer surfaces in an airtight concrete cell at the Waste Safety TEsting Facility (WASTEF) of Nuclear Science Research Institute was modified to enable electrochemical measurements under gamma-ray irradiation. The effect of gamma-rays on the corrosion reaction taking place on the stainless steel surface was discussed from the electrochemical test results obtained. As a result, changes in the immersion potentials of stainless steel and the polarization curves due to chemical species caused by radiolysis of gamma-ray irradiation were confirmed.
 Np
NpIrisawa, Eriko; Kato, Chiaki; Yamashita, Naoki; Sano, Naruto
Zairyo To Kankyo, 71(3), p.70 - 74, 2022/03
In order to evaluate the corrosion of stainless steels used in spent nuclear fuel reprocessing facilities, the immersion corrosion tests and polarization measurements were performed using R-SUS304ULC stainless steel in nitric acid solution containing a kind of radionuclides,  Np. At temperatures above 328 K, the corrosion potential was higher than that in nitric acid solution and was near the transpassive region. From the comparison between the corrosion amount calculated by the immersion corrosion tests and the polarization resistance, the values of
Np. At temperatures above 328 K, the corrosion potential was higher than that in nitric acid solution and was near the transpassive region. From the comparison between the corrosion amount calculated by the immersion corrosion tests and the polarization resistance, the values of 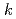 =0.018-0.025 V were obtained as a conversion factor, and the possibility of calculating the corrosion amount from the electrochemical measurement was examined.
=0.018-0.025 V were obtained as a conversion factor, and the possibility of calculating the corrosion amount from the electrochemical measurement was examined.
Yamamoto, Masahiro; Irisawa, Eriko; Igarashi, Takahiro; Komatsu, Atsushi; Kato, Chiaki; Ueno, Fumiyoshi
Proceedings of Annual Congress of the European Federation of Corrosion (EUROCORR 2019) (Internet), 5 Pages, 2019/09
Intergranular corrosion phenomena were analysed using modified reprocessing solution. The data indicated that corrosion rates increased with time at the initial stage, and these stayed at constant value. Intergranular corrosion propagated at grain boundary in the initial stage and then attacked whole grain boundary causing drop out of grains. Corrosion rates of steady state were sum of intergranular corrosion amounts and weight losses of dropped grains. Surface appearances and cross sections of corroded samples were analyzed. The results indicated that the initial stage of intergranular corrosion was characterized by the ratio of corrosion rates between grain boundary and matrix. These ratios differed from individual grain boundaries. Total corrosion rates were affected by the distribution of these ratios. These data were based on the numerical modelling of intergranular corrosion using cellular automata. And also, calculated results were compared with these analytical data.
Irisawa, Eriko; Yamamoto, Masahiro; Kato, Chiaki; Motooka, Takafumi; Ban, Yasutoshi
Journal of Nuclear Science and Technology, 56(4), p.337 - 344, 2019/04
Times Cited Count:6 Percentile:48.99(Nuclear Science & Technology)Kondo, Masatoshi*; Okubo, Nariaki; Irisawa, Eriko; Komatsu, Atsushi; Ishikawa, Norito; Tanaka, Teruya*
Energy Procedia, 131, p.386 - 394, 2017/12
Times Cited Count:6 Percentile:95.25(Energy & Fuels)The chemical behaviors of lead (Pb) based coolants in the air ingress accident of fast reactors were investigated by means of the thermodynamic considerations and the static oxidation experiments for Pb alloys at various chemical compositions. The results of the static oxidation tests for lead-bismuth (Pb-Bi) alloys indicated that Pb was depleted from the alloy due to the preferential formation of PbO in air at 773K. Pb-Bi oxide and Bi O
O were formed after the enrichment of Bi in the alloys due to the Pb depletion. The oxidation rates of the alloys were much larger than that of the steels, and became larger with higher Pb concentration in the alloys. The compatibility of Pb-Bi alloys with stainless steel was worse when the Pb concentration in the alloys became low, since the dissolution type corrosion was promoted by the Bi composition in the alloy. The Pb-Li alloys were oxidized as they formed Li
were formed after the enrichment of Bi in the alloys due to the Pb depletion. The oxidation rates of the alloys were much larger than that of the steels, and became larger with higher Pb concentration in the alloys. The compatibility of Pb-Bi alloys with stainless steel was worse when the Pb concentration in the alloys became low, since the dissolution type corrosion was promoted by the Bi composition in the alloy. The Pb-Li alloys were oxidized as they formed Li PbO
PbO and Li
and Li CO
CO . Then, Li was depleted from the alloy.
. Then, Li was depleted from the alloy.
Irisawa, Eriko; Kato, Chiaki; Kamoshida, Michio*; Hakamatsuka, Yasuyuki*; Ueno, Fumiyoshi; Yamamoto, Masahiro
Proceedings of European Corrosion Congress 2017 (EUROCORR 2017) and 20th ICC & Process Safety Congress 2017 (USB Flash Drive), 9 Pages, 2017/09
Matsueda, Makoto; Irisawa, Eriko; Kato, Chiaki; Matsui, Hiroki
Proceedings of 54th Annual Meeting of Hot Laboratories and Remote Handling (HOTLAB 2017) (Internet), 4 Pages, 2017/00
In the PUREX method, spent fuels are dissolved with nitric acid media. The reprocessing solution containing Fission Products derived from spent fuels is very corrosive to metal materials, the corrosion problem often appears on the surface stainless steel devices. The oxidizing metal ions such as Ruthenium (Ru) and Neptunium (Np) in the process solution is the key reason for severe corrosion of stainless steel. In order to obtain the corrosion rate of stainless steel, we installed the corrosion test apparatus inside an airtight concrete cell in a hot laboratory (the WAste Safety TEsting Facility (WASTEF) of the Japan Atomic Energy Agency), and performed the corrosion tests of stainless steel in the heated nitric acid solution containing Np. The corrosion tests were performed in the temperature range from room temperature to boiling point for 500 hours per batch. The results show that the presence of Np accelerate the stainless steel corrosion in the nitric acid solution.
Ueno, Fumiyoshi; Irisawa, Eriko; Kato, Chiaki; Igarashi, Takahiro; Yamamoto, Masahiro; Abe, Hitoshi
Proceedings of European Corrosion Congress 2016 (EUROCORR 2016) (USB Flash Drive), 7 Pages, 2016/09
In this study, we focused on the effect of the boiling of nitric acid solution on the corrosion of a stainless steel-made concentrator in reduced pressure in fuel reprocessing plant. In order to perform the simulation test in a non-radioactive condition, nitric acid solution with the addition of vanadium as an oxidizing metal ion were used. Corrosion tests were carried out under the conditions of boiling at reduced pressure, and of non-boiling at normal pressure and several temperatures. As a result, corrosion was accelerated by the solution boiling while it was not by non-boiling at the same temperature. It was found also that the temperature dependence of corrosion rate is the same in the both conditions of boiling and non-boiling. The corrosion accelerating effect will be discussed on the basis of the reaction among nitric acid, NOx and vanadium, etc.
Irisawa, Eriko; Ueno, Fumiyoshi; Kato, Chiaki; Abe, Hitoshi
Zairyo To Kankyo, 65(4), p.134 - 137, 2016/04
In order to investigate the effect of boiling under reduced pressure on corrosion of stainless steel in the nitric acid solution, the corrosion tests simulating the high-level radioactive liquid waste evaporator were performed. The results of immersion tests of stainless steels in the solution with and without boiling showed that the corrosion rates in boiling solution were larger than those in not boiling solution in case of same temperature of solution. Moreover, the cathode polarization curves showed that the corrosion potential of stainless steel in boiling solutions were shifted nobler, and the current intensity became larger than that in not boiling solutions. According to these results, it can be concluded that boiling of solution under reduced pressure accelerate the corrosion rates.
Irisawa, Eriko; Seki, Masaharu*; Ueno, Fumiyoshi; Kato, Chiaki; Motooka, Takafumi; Abe, Hitoshi
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.1108 - 1112, 2015/09
Irisawa, Eriko; Ueno, Fumiyoshi; Uchida, Naoki; Taguchi, Katsuya
Proceedings of 23rd International Conference on Nuclear Engineering (ICONE-23) (DVD-ROM), 3 Pages, 2015/05
Irisawa, Eriko; Ueno, Fumiyoshi; Yamamoto, Masahiro; Uchida, Naoki; Taguchi, Katsuya
no journal, ,
The effect of seawater added in the boiling nitric acid solution on the corrosion of steel used in the spent fuel reprocessing facility was investigated. The corrosion rates of 310 type austenitic stainless steel in the boiling nitric acid with containing of vanadium and chloride ion were obtained by the immersion corrosion tests under ordinary pressure. In case of the presence of the oxidizing cation (vanadium in this study) and the composition of seawater in the nitric acid solution, the corrosion rates were decreased compared with the case the absence of seawater.
Ueno, Fumiyoshi; Irisawa, Eriko; Abe, Hitoshi
no journal, ,
The reprocessing components made of stainless steel appear intergranular corrosion in boiling nitric acid solution containing high oxidizing metal ions derived from spent nuclear fuel. In this paper, to clarify the corrosion mechanism in this environment, temperature dependence of corrosion rate of a stainless steel in boiling 3 molar nitric acid with added pentavalent vanadium was studied. As the results of investigation of the temperature dependence of the corrosion rate of the heat transfer surface using estimated temperature from a boiling curve, it was found that there was no effect of heat flux on corrosion rate because the dependence was consistent between heat transfer condition and non-heat-transfer. Also it was found that the activation energy was different between the boiling conditions of about 378 K or more and the non-boiling conditions of below 378 K.
Irisawa, Eriko; Ueno, Fumiyoshi; Abe, Hitoshi; Kumagai, Mikio*; Suzuki, Kazunori*; Hayashi, Shinichiro*
no journal, ,
no abstracts in English
Ueno, Fumiyoshi; Irisawa, Eriko; Seki, Masaharu; Abe, Hitoshi
no journal, ,
no abstracts in English
Irisawa, Eriko; Ueno, Fumiyoshi; Seki, Masaharu; Abe, Hitoshi
no journal, ,
no abstracts in English
Irisawa, Eriko; Ueno, Fumiyoshi; Abe, Hitoshi
no journal, ,
no abstracts in English
Igarashi, Takahiro; Kato, Chiaki; Irisawa, Eriko; Ueno, Fumiyoshi
no journal, ,
It is known that corrosion rate of stainless steel in nitric acid solution is affected by the valence change of oxidizing metallic ions. In this study, we conducted redox reaction analyses using chemical reaction calculation model to clarify the mechanism of valence change. We obtained that the oxidization of metallic ions in the solution is not only caused by nitrus acid but also nitrogen oxides.
Igarashi, Takahiro; Irisawa, Eriko; Kato, Chiaki; Ueno, Fumiyoshi; Abe, Hitoshi
no journal, ,
It is known that existence of oxidizing metal ion in boiling nitric acid solution makes stainless steel more corrosive. In this study, in order to elucidate chemical species which promote oxidization of Vanadium(IV) ion, we conducted Redox reaction simulation in nitric acid solution using chemical reaction model. The results showed that nitrous acid and nitrogen dioxide affected oxidization of Vanadium(IV) ion, and rate constant of nitrogen dioxide was larger than that of nitrous acid.