Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 C
CPham, V. H.; Kurata, Masaki; Nagae, Yuji; Ishibashi, Ryo*; Sasaki, Masana*
Corrosion Science, 255, p.113098_1 - 113098_9, 2025/10
Times Cited Count:0Ishibashi, Ryo*; Hirosaka, Kazuma*; Yamana, Takashi*; Shibata, Masatoshi*; Sasaki, Masana*; Nemoto, Yoshiyuki; Hinoki, Tatsuya*
Proceedings of TopFuel 2024 (Internet), 9 Pages, 2024/09
Pham, V. H.; Nagae, Yuji; Kurata, Masaki; Furumoto, Kenichiro*; Sato, Hisaki*; Ishibashi, Ryo*; Yamashita, Shinichiro
Proceedings of International Nuclear Fuel Cycle Conference / Light Water Reactor Fuel Performance Conference (Global/Top Fuel 2019) (USB Flash Drive), p.670 - 674, 2019/09
Ishibashi, Ryo*; Tanabe, Shigetada*; Kondo, Takao*; Yamashita, Shinichiro; Nagase, Fumihisa
Proceedings of 2017 Water Reactor Fuel Performance Meeting (WRFPM 2017) (USB Flash Drive), 10 Pages, 2017/09
For improving the corrosion resistance of silicon carbide (SiC) in boiling-water-reactor environments, corrosion-resistant coatings on SiC were evaluated. Due to its hydrogen-generation rate and reaction heat being lower than those of conventional Zircaloy, SiC is expected to be an appropriate material for accident-tolerant fuels. However, there are still many critical issues with the practical application of SiC fuel cladding and fuel channel boxes, one of which is hydrothermal corrosion. Silicon carbide is chemically stable, but silicon oxide formed by oxidation of SiC dissolves in high temperature water. Although the rate of SiC dissolution is very small, the dissolution must be suppressed to comply with regulations for dissolved silica concentration in reactor coolant. In this study, the corrosion behavior of candidate coatings for SiC substrates were evaluated before and after exposure to unirradiated high-purity-water environments.
 binding site of a
binding site of a  -lactamase from the halophile by anomalous X-ray diffraction
-lactamase from the halophile by anomalous X-ray diffractionArai, Shigeki; Shibazaki, Chie; Shimizu, Rumi; Adachi, Motoyasu; Tamada, Taro; Tokunaga, Hiroko*; Ishibashi, Matsujiro*; Tokunaga, Masao*; Kuroki, Ryota
Kyushu Shinkurotoronko Kenkyu Senta Nempo, 2014, p.17 - 19, 2016/03
no abstracts in English
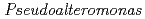 sp. AS-131 isolated from Antarctic Ocean
sp. AS-131 isolated from Antarctic OceanYonezawa, Yasushi*; Nagayama, Aiko*; Tokunaga, Hiroko*; Ishibashi, Matsujiro*; Arai, Shigeki; Kuroki, Ryota; Watanabe, Keiichi*; Arakawa, Tsutomu*; Tokunaga, Masao*
Protein Journal, 34(4), p.275 - 283, 2015/08
Times Cited Count:4 Percentile:9.94(Biochemistry & Molecular Biology)Nucleoside diphosphate kinase isolated from psychrophilic 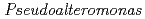 sp. AS-131 (ASNDK) was expressed in
sp. AS-131 (ASNDK) was expressed in  and purified to homogeneity. Comparing to mesophilic NDK isolated from
and purified to homogeneity. Comparing to mesophilic NDK isolated from 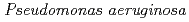 , ASNDK exhibited highly elevated thermolability: (1)
, ASNDK exhibited highly elevated thermolability: (1) 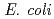 expression at 37
expression at 37 C as a denatured insoluble form, and (2) 30
C as a denatured insoluble form, and (2) 30 C lower optimum temperature of enzymatic activity. The subunit structure of ASNDK was suggested to be dimer, as in NDKs isolated from moderate halophiles.
C lower optimum temperature of enzymatic activity. The subunit structure of ASNDK was suggested to be dimer, as in NDKs isolated from moderate halophiles.
 -lactamase from the moderate halophile
-lactamase from the moderate halophile  sp.560 and the discovery of a Cs
sp.560 and the discovery of a Cs -selective binding site
-selective binding siteArai, Shigeki; Yonezawa, Yasushi*; Okazaki, Nobuo*; Matsumoto, Fumiko*; Shibazaki, Chie; Shimizu, Rumi; Yamada, Mitsugu*; Adachi, Motoyasu; Tamada, Taro; Kawamoto, Masahide*; et al.
Acta Crystallographica Section D, 71(3), p.541 - 554, 2015/03
Times Cited Count:8 Percentile:52.42(Biochemical Research Methods)The crystal structure of halophilic  -lactamase from
-lactamase from  sp.560 (HaBLA) was determined using X-ray crystallography. Moreover, the locations of bound Sr
sp.560 (HaBLA) was determined using X-ray crystallography. Moreover, the locations of bound Sr and Cs
and Cs ions were identified by anomalous X-ray diffraction. The location of one Cs
ions were identified by anomalous X-ray diffraction. The location of one Cs specific binding site was identified on HaBLA even in the presence of 9-fold molar excess of Na
specific binding site was identified on HaBLA even in the presence of 9-fold molar excess of Na (90 mM Na
(90 mM Na /10 mM Cs
/10 mM Cs ). This Cs
). This Cs binding site is formed by two main-chain O atoms and an aromatic ring of a side chain of Trp. An aromatic ring of Trp interacts with Cs
binding site is formed by two main-chain O atoms and an aromatic ring of a side chain of Trp. An aromatic ring of Trp interacts with Cs by the cation-
by the cation- interaction. The observation of a selective and high-affinity Cs
interaction. The observation of a selective and high-affinity Cs binding site provides important information that is useful for designing artificial Cs
binding site provides important information that is useful for designing artificial Cs binding sites useful in bioremediation of radioactive isotopes.
binding sites useful in bioremediation of radioactive isotopes.
 sp.593
sp.593Arai, Shigeki; Yonezawa, Yasushi*; Ishibashi, Matsujiro*; Matsumoto, Fumiko*; Adachi, Motoyasu; Tamada, Taro; Tokunaga, Hiroko*; Blaber, M.; Tokunaga, Masao*; Kuroki, Ryota
Acta Crystallographica Section D, 70(3), p.811 - 820, 2014/03
Times Cited Count:13 Percentile:63.41(Biochemical Research Methods)In order to clarify the structural basis of halophilic characteristics of an alkaline phosphatase derived from the moderate halophile  sp.593 (HaAP), the tertiary structure of HaAP was determined to 2.1
sp.593 (HaAP), the tertiary structure of HaAP was determined to 2.1 resolution by X-ray crystallography. Structural properties of surface negative charge and core hydrophobicity are shown to be intermediate between halophile and non-halophile characteristics, and may explain the unique functional adaptation to a wide-range of salt concentration.
resolution by X-ray crystallography. Structural properties of surface negative charge and core hydrophobicity are shown to be intermediate between halophile and non-halophile characteristics, and may explain the unique functional adaptation to a wide-range of salt concentration.
Hama, Katsuhiro; Mikake, Shinichiro; Nishio, Kazuhisa; Matsuoka, Toshiyuki; Ishibashi, Masayuki; Sasao, Eiji; Hikima, Ryoichi*; Tanno, Takeo*; Sanada, Hiroyuki; Onoe, Hironori; et al.
JAEA-Review 2013-050, 114 Pages, 2014/02
Japan Atomic Energy Agency (JAEA) at Tono Geoscience Center (TGC) is pursuing a geoscientific research and development project namely the Mizunami Underground Research Laboratory (MIU) Project in crystalline rock environment in order to construct scientific and technological basis for geological disposal of High-level Radioactive Waste (HLW). The MIU Project has three overlapping phases: Surface-based Investigation phase (Phase I), Construction phase (Phase II), and Operation phase (Phase III). The MIU Project has been ongoing the Phase II and the Phase III in fiscal year 2012. This report presents the results of the investigations, construction and collaboration studies in fiscal year 2012, as a part of the Phase II and Phase III based on the MIU Master Plan updated in 2010.
Kunimaru, Takanori; Mikake, Shinichiro; Nishio, Kazuhisa; Tsuruta, Tadahiko; Matsuoka, Toshiyuki; Ishibashi, Masayuki; Sasao, Eiji; Hikima, Ryoichi; Tanno, Takeo; Sanada, Hiroyuki; et al.
JAEA-Review 2013-018, 169 Pages, 2013/09
Japan Atomic Energy Agency (JAEA) at Tono Geoscience Center (TGC) is pursuing a geoscientific research and development project namely the Mizunami Underground Research Laboratory (MIU) Project in crystalline rock environment in order to construct scientific and technological basis for geological disposal of High-level Radioactive Waste (HLW). The MIU Project has three overlapping phases: Surface-based Investigation phase (Phase I), Construction phase (Phase II), and Operation phase (Phase III). The MIU Project has been ongoing the Phase II and the Phase III in 2011 fiscal year. This report shows the results of the investigation, construction and collaboration studies in fiscal year 2011, as a part of the Phase II and Phase III based on the MIU Master Plan updated in 2010.
Ishibashi, Matsujiro*; Uchino, Manami*; Arai, Shigeki; Kuroki, Ryota; Arakawa, Tsutomu*; Tokunaga, Masao*
Archives of Biochemistry and Biophysics, 525(1), p.47 - 52, 2012/09
Times Cited Count:7 Percentile:19.80(Biochemistry & Molecular Biology)Nucleoside diphosphate kinase (HsNDK) from Halobacterium salinarum requires salt at high concentrations for folding. A D148C mutant, in which Asp148 was replaced with Cys, was designed to enhance stability and folding in low salt solution by S-S bond. It showed increased thermal stability by about 10 C in 0.2 M NaCl over the wild type HsNDK. It refolded from heat-denaturation even in 0.1 M NaCl, while the wild type required 2 M NaCl to achieve the same level of activity recovery. This enhanced refolding is due to the three S-S bonds between two basic dimeric units in the hexameric HsNDK structure. Moreover, salt concentration dependency of heat-denaturation process and refolding process of the wild type and D148C mutant HsNDKs were investigated by the circular dichroism and native-PAGE analysis.
C in 0.2 M NaCl over the wild type HsNDK. It refolded from heat-denaturation even in 0.1 M NaCl, while the wild type required 2 M NaCl to achieve the same level of activity recovery. This enhanced refolding is due to the three S-S bonds between two basic dimeric units in the hexameric HsNDK structure. Moreover, salt concentration dependency of heat-denaturation process and refolding process of the wild type and D148C mutant HsNDKs were investigated by the circular dichroism and native-PAGE analysis.
Kunimaru, Takanori; Mikake, Shinichiro; Nishio, Kazuhisa; Tsuruta, Tadahiko; Matsuoka, Toshiyuki; Ishibashi, Masayuki; Kuboshima, Koji; Takeuchi, Ryuji; Mizuno, Takashi; Sato, Toshinori; et al.
JAEA-Review 2012-028, 31 Pages, 2012/08
Japan Atomic Energy Agency (JAEA) at Tono Geoscience Center (TGC) is pursuing a geoscientific research and development project namely the Mizunami Underground Research Laboratory (MIU) project in crystalline rock environment in order to construct scientific and technological basis for geological disposal of High-level Radioactive Waste (HLW). The MIU project is planned in three overlapping phases; Surface-based Investigation Phase (Phase I), Construction Phase (Phase II) and Operation Phase (Phase III). Currently, the project is under the Construction Phase and the Operation Phase. This document introduces the research and development activities planned for 2012 fiscal year based on the MIU Master Plan updated in 2010, construction plan and research collaboration plan, etc.
Kunimaru, Takanori; Mikake, Shinichiro; Nishio, Kazuhisa; Tsuruta, Tadahiko; Matsuoka, Toshiyuki; Ishibashi, Masayuki; Ueno, Takashi; Tokuyasu, Shingo; Daimaru, Shuji; Takeuchi, Ryuji; et al.
JAEA-Review 2012-020, 178 Pages, 2012/06
Japan Atomic Energy Agency (JAEA) at Tono Geoscience Center (TGC) is pursuing a geoscientific research and development project namely the Mizunami Underground Research Laboratory (MIU) Project in crystalline rock environment in order to construct scientific and technological basis for geological disposal of High-level Radioactive Waste (HLW). The MIU Project has three overlapping phases: Surface-based Investigation phase (Phase I), Construction phase (Phase II), and Operation phase (Phase III). The MIU Project has been ongoing the Phase II. And Phase III started in 2010 fiscal year. This report shows the results of the investigation, construction and collaboration studies in fiscal year 2010, as a part of the Phase II based on the MIU Master Plan updated in 2002.
 -sheet-rich prion protein in scrapie-infected neuroblastoma cells with human IgG1 antibody specific for
-sheet-rich prion protein in scrapie-infected neuroblastoma cells with human IgG1 antibody specific for  -form prion protein
-form prion proteinKubota, Toshiya*; Hamazoe, Yuta*; Hashiguchi, Shuhei*; Ishibashi, Daisuke*; Akasaka, Kazuyuki*; Nishida, Noriyuki*; Katamine, Shigeru*; Sakaguchi, Suehiro*; Kuroki, Ryota; Nakashima, Toshihiro*; et al.
Journal of Biological Chemistry, 287(17), p.14023 - 14039, 2012/04
Times Cited Count:5 Percentile:10.88(Biochemistry & Molecular Biology)We prepared  -sheet-rich recombinant full-length prion protein (
-sheet-rich recombinant full-length prion protein ( -form PrP) (Jackson, G. S., Hosszu, L. L., Power, A., Hill, A. F., Kenney, J., Saibil, H., Craven, C. J., Waltho, J. P., Clarke, A. R., and Collinge, J. (1999) Science 283, 1935-1937). Using this
-form PrP) (Jackson, G. S., Hosszu, L. L., Power, A., Hill, A. F., Kenney, J., Saibil, H., Craven, C. J., Waltho, J. P., Clarke, A. R., and Collinge, J. (1999) Science 283, 1935-1937). Using this  -form PrP and a human single chain Fv-displaying phage library, we have established a human IgG1 antibody specific to
-form PrP and a human single chain Fv-displaying phage library, we have established a human IgG1 antibody specific to  -form but not
-form but not  -form PrP, PRB7 IgG. When prion-infected ScN2a cells were cultured with PRB7 IgG, they generated and accumulated PRB7-binding granules in the cytoplasm with time, consequently becoming apoptotic cells bearing very large PRB7-bound aggregates. The SAF32 antibody recognizing the N-terminal octarepeat region of full-length PrP stained distinct granules in these cells as determined by confocal laser microscopy observation. When the accumulation of proteinase K-resistant PrP was examined in prion-infected ScN2a cells cultured in the presence of PRB7 IgG or SAF32, it was strongly inhibited by SAF32 but not at all by PRB7 IgG. Thus, we demonstrated direct evidence of the generation and accumulation of
-form PrP, PRB7 IgG. When prion-infected ScN2a cells were cultured with PRB7 IgG, they generated and accumulated PRB7-binding granules in the cytoplasm with time, consequently becoming apoptotic cells bearing very large PRB7-bound aggregates. The SAF32 antibody recognizing the N-terminal octarepeat region of full-length PrP stained distinct granules in these cells as determined by confocal laser microscopy observation. When the accumulation of proteinase K-resistant PrP was examined in prion-infected ScN2a cells cultured in the presence of PRB7 IgG or SAF32, it was strongly inhibited by SAF32 but not at all by PRB7 IgG. Thus, we demonstrated direct evidence of the generation and accumulation of  -sheet-rich PrP in ScN2a cells de novo. These results suggest first that PRB7-bound PrP is not responsible for the accumulation of
-sheet-rich PrP in ScN2a cells de novo. These results suggest first that PRB7-bound PrP is not responsible for the accumulation of  -form PrP aggregates, which are rather an end product resulting in the triggering of apoptotic cell death, and second that SAF32-bound PrP lacking the PRB7-recognizing
-form PrP aggregates, which are rather an end product resulting in the triggering of apoptotic cell death, and second that SAF32-bound PrP lacking the PRB7-recognizing  -form may represent so-called PrPSc with prion propagation activity. PRB7 is the first human antibody specific to
-form may represent so-called PrPSc with prion propagation activity. PRB7 is the first human antibody specific to  -form PrP and has become a powerful tool for the characterization of the biochemical nature of prion and its pathology.
-form PrP and has become a powerful tool for the characterization of the biochemical nature of prion and its pathology.
 sp. nucleoside diphosphate kinase
sp. nucleoside diphosphate kinaseArai, Shigeki; Yonezawa, Yasushi; Okazaki, Nobuo; Matsumoto, Fumiko; Tamada, Taro; Tokunaga, Hiroko*; Ishibashi, Matsujiro*; Blaber, M.; Tokunaga, Masao*; Kuroki, Ryota
Protein Science, 21(4), p.498 - 510, 2012/04
Times Cited Count:15 Percentile:34.81(Biochemistry & Molecular Biology)In order to clarify the oligomer state of nucleoside diphosphate kinase (NDK) from moderately halophilic  sp. 593 (HaNDK), the crystal structure of HaNDK was determined by X-ray crystallography. The crystal structures of the wild-type HaNDK and the mutant HaNDK (E134A) showed a dimer and a tetramer, respectively. The higher ordered association of proteins usually contributes to an increase in thermal stability and substrate affinity. The change in the assembly form by a minimum mutation may be an effective way for NDK to acquire molecular characteristics suited to various circumstances.
sp. 593 (HaNDK), the crystal structure of HaNDK was determined by X-ray crystallography. The crystal structures of the wild-type HaNDK and the mutant HaNDK (E134A) showed a dimer and a tetramer, respectively. The higher ordered association of proteins usually contributes to an increase in thermal stability and substrate affinity. The change in the assembly form by a minimum mutation may be an effective way for NDK to acquire molecular characteristics suited to various circumstances.
Tokunaga, Hiroko*; Ishibashi, Matsujiro*; Arisaka, Fimio*; Arai, Shigeki; Kuroki, Ryota; Arakawa, Tsutomu*; Tokunaga, Masao*
FEBS Letters, 582(7), p.1049 - 1054, 2008/04
Times Cited Count:17 Percentile:39.33(Biochemistry & Molecular Biology) nucleoside diphosphate kinase (HaNDK) forms a dimeric assembly and
nucleoside diphosphate kinase (HaNDK) forms a dimeric assembly and 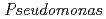 NDK (PaNDK) forms a tetrameric assembly. The mutation of Glu134 to Ala in HaNDK resulted in conversion of the native dimeric structure to the tetramer assembly. Conversely, the mutation of Ala134 to Glu in PaNDK leads to conversion from tetramer to dimer assembly, indicating that a single amino acid substitution at position 134 results in an alteration of the oligomeric structure of NDK. Modeling structure of HaNDK and PaNDK, based on the crystal structure of
NDK (PaNDK) forms a tetrameric assembly. The mutation of Glu134 to Ala in HaNDK resulted in conversion of the native dimeric structure to the tetramer assembly. Conversely, the mutation of Ala134 to Glu in PaNDK leads to conversion from tetramer to dimer assembly, indicating that a single amino acid substitution at position 134 results in an alteration of the oligomeric structure of NDK. Modeling structure of HaNDK and PaNDK, based on the crystal structure of 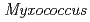 NDK, suggested sufficient repulsion by Glu134 to disrupt dimer-dimer interaction to form tetramer.
NDK, suggested sufficient repulsion by Glu134 to disrupt dimer-dimer interaction to form tetramer.
Tamada, Taro; Honjo, Eijiro; Maeda, Yoshitake*; Okamoto, Tomoyuki*; Ishibashi, Matsujiro*; Tokunaga, Masao*; Kuroki, Ryota
Proceedings of the National Academy of Sciences of the United States of America, 103(9), p.3135 - 3140, 2006/02
Times Cited Count:99 Percentile:84.85(Multidisciplinary Sciences)A crystal structure of the signaling complex between human granulocyte colony-stimulating factor (GCSF), and a ligand binding region of GCSF receptor (GCSF-R), has been determined to 2.8 resolution. The GCSF:GCSF-R complex formed a 2:2 stoichiometry via a cross-over interaction between the Ig-like domains of GCSF-R and GCSF. The conformation of the complex is quite different to that between human GCSF and the CRH domain of mouse GCSF-R, but similar to that of the interleukin-6 (IL-6)/gp130 signaling complex. The Ig-like domain cross-over structure necessary for GCSF-R activation is consistent with previously reported thermodynamic and mutational analyses.
resolution. The GCSF:GCSF-R complex formed a 2:2 stoichiometry via a cross-over interaction between the Ig-like domains of GCSF-R and GCSF. The conformation of the complex is quite different to that between human GCSF and the CRH domain of mouse GCSF-R, but similar to that of the interleukin-6 (IL-6)/gp130 signaling complex. The Ig-like domain cross-over structure necessary for GCSF-R activation is consistent with previously reported thermodynamic and mutational analyses.
Honjo, Eijiro; Tamada, Taro; Maeda, Yoshitake*; Koshiba, Takumi*; Matsukura, Yasuko*; Okamoto, Tomoyuki*; Ishibashi, Matsujiro*; Tokunaga, Masao*; Kuroki, Ryota
Acta Crystallographica Section F, 61(8), p.788 - 790, 2005/08
Times Cited Count:7 Percentile:54.61(Biochemical Research Methods)Granulocyte colony-stimulating factor (GCSF) receptor receives signals for regulating the proliferation and differentiation of the precursor cells of granulocytes. The complex composed of two GCSFs and two GCSF receptors was crystallized. The crystal of the complex was grown in 1.0 M sodium formate and 0.1 M sodium acetate (pH4.6). It belongs to the space group  4
4 2
2 2 (or its enantiomorph
2 (or its enantiomorph  4
4 2
2 2) with unit cell dimensions of
2) with unit cell dimensions of  =
=  = 110.1
= 110.1  ,
,  = 331.8
= 331.8  . However, the diffraction data from the crystal beyond 5
. However, the diffraction data from the crystal beyond 5  resolution could not be collected. Since the heterogeneity of GCSF receptor seems to interrupt growth of good quality crystals, the GCSF receptor was fractionated by achromatography. Crystals of GCSF/fractionated GCSF receptor complex were grown as a new crystal form in 0.2 M ammonium phosphate. The new crystal diffracts beyond 3.0
resolution could not be collected. Since the heterogeneity of GCSF receptor seems to interrupt growth of good quality crystals, the GCSF receptor was fractionated by achromatography. Crystals of GCSF/fractionated GCSF receptor complex were grown as a new crystal form in 0.2 M ammonium phosphate. The new crystal diffracts beyond 3.0  resolution and belongs to space group
resolution and belongs to space group  3
3 21 (or its enantiomorph
21 (or its enantiomorph  3
3 21) with unit-cell parameters
21) with unit-cell parameters  =
=  = 134.8,
= 134.8,  = 105.7
= 105.7  .
.
 sp.593 (HaNDK)
sp.593 (HaNDK)Arai, Shigeki; Yonezawa, Yasushi; Okazaki, Nobuo; Tamada, Taro; Tokunaga, Hiroko*; Ishibashi, Matsujiro*; Tokunaga, Masao*; Kuroki, Ryota
no journal, ,
The nucleoside diphosphate kinases (NDKs) are known to have a tetrameric or hexameric oligomer structure formed by association of common dimeric components. In order to understand the oligomeric structure of NDK, the wild-type NDK from Halomonas sp. 593 (HaNDK) was determined by X-ray crystallography. The wild-type HaNDK only exhibited a dimeric form that is the same as a common dimer unit seen in other NDKs. This is the first evidence of a dimeric structure for HaNDK. To explore the effect of mutation on the assembly form of HaNDK, E134, which is a key interface residue for other tetrameric NDKs, was replaced with Ala, and the structure was determined by X-ray crystallography. Two kinds of tetrameric assemblies, Type I and Type II, appeared in the asymmetric unit of the E134A mutant HaNDK. The change from dimeric to tetrameric assembly is attributed to the removal of negative charge repulsion caused by the E134 in the wild-type HaNDK.
Arai, Shigeki; Yonezawa, Yasushi; Okazaki, Nobuo; Tamada, Taro; Tokunaga, Hiroko*; Ishibashi, Matsujiro*; Tokunaga, Masao*; Kuroki, Ryota
no journal, ,
The nucleoside diphosphate kinases (NDKs) are known to have a tetrameric or hexameric oligomer structure formed by association of common dimeric components. We determined the crystal structure of E134A mutant NDK from Halomonas sp. 593 (HaNDK) and found that two kinds of tetrameric assemblies, Type I seen in the Myxococcus NDK tetramer and Type II seen in the E.coli NDK tetramer, appeared in the asymmetric unit. Change in the assembly form may be an effective way for NDK to acquire molecular characteristics suited to various circumstances.