Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Watanabe, Takahiro; Ishii, Chikako; Ishizaka, Chika; Niwa, Masakazu; Shimada, Koji; Sawai, Yuki*; Tsuchiya, Noriyoshi*; Matsunaka, Tetsuya*; Ochiai, Shinya*; Nara, Fumiko*
Journal of Mineralogical and Petrological Sciences, 116(3), p.140 - 158, 2021/00
Times Cited Count:5 Percentile:32.44(Mineralogy)A portable energy dispersive X-ray fluorescence spectrometer (potable XRF) can be an effective tool for detecting chemical elements in various materials, such as geological, and environmental samples. In this study, working curves were confirmed using reference materials, such as igneous rocks and other geochemical standards, distributed by national and international organizations. Subsequently, quantification and semi-quantification analyses were performed by the portable XRF for inorganic elements in (A) fault rocks, (B) lake sediments from the middle Japan, and (C) soils with paleotsunami deposits from the Pacific coast of northeast Japan. Twenty-four elements (Mg-U) in these geological samples were measured by potable XRF using our working curves. Measured values by the portable XRF of the samples were good agreement with the reported values in almost cases.
Tateishi, Ryo*; Shimada, Koji; Niwa, Masakazu; Sueoka, Shigeru; Shimizu, Mayuko; Kanno, Mizuho; Ishii, Chikako; Ishimaru, Tsuneari
no journal, ,
The major difference between active faults and inactive faults is the elapsed time after the latest activity, and while active faults are considered to be on the order of 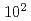 to
to 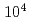 years, inactive faults are over
years, inactive faults are over 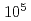 years. Therefore, even if the phenomena caused by fault activity are the same in both cases, the chemical changes that occur during the subsequent rest period of fault activity may differ significantly. In this study, the chemical composition of fault clay was collected by literature values and actual analysis, and the feasibility of discrimination by the chemical composition examined by linear discriminant analysis. According to the 11 elements selected based on the AIC, 45 active fault samples and 51 inactive fault samples were identified with a discrimination rate of 96%. Among the elements, TiO
years. Therefore, even if the phenomena caused by fault activity are the same in both cases, the chemical changes that occur during the subsequent rest period of fault activity may differ significantly. In this study, the chemical composition of fault clay was collected by literature values and actual analysis, and the feasibility of discrimination by the chemical composition examined by linear discriminant analysis. According to the 11 elements selected based on the AIC, 45 active fault samples and 51 inactive fault samples were identified with a discrimination rate of 96%. Among the elements, TiO and P
and P O
O tended to be concentrated as the latest activity period was newer. These concentration mechanisms are for future work.
tended to be concentrated as the latest activity period was newer. These concentration mechanisms are for future work.