Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kosugi, Yoshihisa*; Goto, Matato*; Tan, Z.*; Kan, Daisuke*; Isobe, Masahiko*; Yoshii, Kenji; Mizumaki, Masaichiro*; Fujita, Asaya*; Takagi, Hidenori*; Shimakawa, Yuichi*
Scientific Reports (Internet), 11(1), p.12682_1 - 12682_8, 2021/06
Times Cited Count:5 Percentile:44.86(Multidisciplinary Sciences)Caloric effects of solids provide more efficient and environment-friendly innovative refrigeration systems compared to the widely-used conventional vapor compressive cooling systems. Exploring novel caloric materials is challenging but critically important in developing future technologies. Here we discovered that the quadruple perovskite structure ferrimagnet BiCu Cr
Cr O
O shows a large multicaloric effect at the first-order charge transition occurred around 190 K. Large latent heat and the corresponding isothermal entropy changes 28.2 J K
shows a large multicaloric effect at the first-order charge transition occurred around 190 K. Large latent heat and the corresponding isothermal entropy changes 28.2 J K kg
kg can be fully utilized by applying both magnetic fields (magnetocaloric effect) and pressure (barocaloric effect). Adiabatic temperature changes reach 3.9 K for the 50 kOe magnetic field and 4.8 K for the 4.9 kbar pressure, and thus highly efficient thermal controls are achieved by multiple ways.
can be fully utilized by applying both magnetic fields (magnetocaloric effect) and pressure (barocaloric effect). Adiabatic temperature changes reach 3.9 K for the 50 kOe magnetic field and 4.8 K for the 4.9 kbar pressure, and thus highly efficient thermal controls are achieved by multiple ways.
Suzuki, Eriko; Takase, Gaku; Nakajima, Kunihisa; Nishioka, Shunichiro; Hashimoto, Naoyuki*; Isobe, Shigehito*; Osaka, Masahiko
Proceedings of International Topical Workshop on Fukushima Decommissioning Research (FDR 2019) (Internet), 4 Pages, 2019/05
In order to acquire the knowledge of the Cs chemisorption behaviour in the lower temperature region, the Cs chemisorbed compounds and the surface reaction rates were investigated by conducting the Cs chemisorption tests onto stainless steel at 873 and 973 K. As a result, The cesium ferrate compounds were revealed to be formed at this temperatures. It was seen that the dependences of surface reaction rate constant on this temperature were different from that at the higher temperature region. This behaviour leads to the conclusion that the Cs chemisorption model in the low temperature region should be newly constructed.

Kodama, Katsuaki; Ikeda, Kazutaka*; Isobe, Masahiko*; Takeda, Hikaru*; Ito, Masayuki*; Ueda, Yutaka*; Shamoto, Shinichi; Otomo, Toshiya*
Journal of the Physical Society of Japan, 85(9), p.094709_1 - 094709_5, 2016/09
Times Cited Count:1 Percentile:11.46(Physics, Multidisciplinary) as the origin of volume collapse
as the origin of volume collapseYu, R.*; Hojo, Hajime*; Watanuki, Tetsu; Mizumaki, Masaichiro*; Mizokawa, Takashi*; Oka, Kengo*; Kim, H.*; Machida, Akihiko; Sakaki, Koji*; Nakamura, Yumiko*; et al.
Journal of the American Chemical Society, 137(39), p.12719 - 12728, 2015/10
Times Cited Count:34 Percentile:70.17(Chemistry, Multidisciplinary)no abstracts in English
 O
O ; Understanding of the details of the Ising spin and the competitive interactions which made the devil's flower bloom
; Understanding of the details of the Ising spin and the competitive interactions which made the devil's flower bloomOwada, Kenji; Fujii, Yasuhiko; Muraoka, Jiro*; Nakao, Hironori*; Murakami, Yoichi*; Noda, Yukio*; Osumi, Hiroyuki*; Ikeda, Naoshi*; Shobu, Takahisa; Isobe, Masahiko*; et al.
Hoshako, 21(2), p.87 - 96, 2008/03
Devil's flower has been found in a temperature-pressure phase diagram of NaV O
O , which shows a charge disproportionation (CD) at ambient pressure. By a complementary use of an X-ray structural analysis and a resonant X-ray diffraction, which is sensitive to CD, we have investigated the structural relationship between two ground states appeared in lower and higher pressure regions including the charge arrangements. It has been clarified that two equivalent types of charge arrangement in CD correspond to the Ising variable in NaV
, which shows a charge disproportionation (CD) at ambient pressure. By a complementary use of an X-ray structural analysis and a resonant X-ray diffraction, which is sensitive to CD, we have investigated the structural relationship between two ground states appeared in lower and higher pressure regions including the charge arrangements. It has been clarified that two equivalent types of charge arrangement in CD correspond to the Ising variable in NaV O
O . The atomic shifts are regarded as linearly coupled to the Ising spins. The results lead us to the conclusion that it is the first time that the devil's flower blooms in a charge-disproportionation system. The results also lead us to a hypothesis that the competitive interactions between a Ising spins may result from the Ising spin-phonon coupling.
. The atomic shifts are regarded as linearly coupled to the Ising spins. The results lead us to the conclusion that it is the first time that the devil's flower blooms in a charge-disproportionation system. The results also lead us to a hypothesis that the competitive interactions between a Ising spins may result from the Ising spin-phonon coupling.
 O
O under high pressure; A Synchrotron X-ray diffraction study
under high pressure; A Synchrotron X-ray diffraction studyOwada, Kenji; Fujii, Yasuhiko; Muraoka, Jiro*; Nakao, Hironori*; Murakami, Yoichi; Noda, Yukio*; Osumi, Hiroyuki*; Ikeda, Naoshi*; Shobu, Takahisa; Isobe, Masahiko*; et al.
Physical Review B, 76(9), p.094113_1 - 094113_10, 2007/09
Times Cited Count:10 Percentile:44.52(Materials Science, Multidisciplinary)Structural relations between two ground states of the ANNNI (Axial Next Nearest Neighbor Ising) compound NaV O
O , C
, C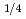 - and C
- and C -phases below and above the transition pressure
-phases below and above the transition pressure 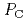 = 1 GPa, were investigated by X-ray diffraction and scattering techniques. The structure of the C
= 1 GPa, were investigated by X-ray diffraction and scattering techniques. The structure of the C -phase is well explained by the
-phase is well explained by the 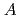 (
(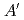 ) pattern which is one of four layers (
) pattern which is one of four layers (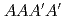 ) of the C
) of the C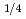 -phase, however, the amount of the atomic shifts under the conditions 1.6 GPa and 6 K is 27% that under ambient pressure. On the other hand, resonant X-ray scattering showed that the charges are disproportionated under high pressure. Based on these facts, it was concluded that charge disproportionation corresponds to the Ising variable in NaV
-phase, however, the amount of the atomic shifts under the conditions 1.6 GPa and 6 K is 27% that under ambient pressure. On the other hand, resonant X-ray scattering showed that the charges are disproportionated under high pressure. Based on these facts, it was concluded that charge disproportionation corresponds to the Ising variable in NaV O
O , where the atomic shifts are regarded as linearly coupled to the Ising spins. These results lead to the hypothesis that the competitive interactions between the Ising spins may result from the Ising spin-phonon coupling.
, where the atomic shifts are regarded as linearly coupled to the Ising spins. These results lead to the hypothesis that the competitive interactions between the Ising spins may result from the Ising spin-phonon coupling.
 O
O
Owada, Kenji; Fujii, Yasuhiko; Nakao, Hironori*; Murakami, Yoichi*; Isobe, Masahiko*; Ueda, Yutaka*
Modern Physics Letters B, 20(5), p.199 - 214, 2006/02
Times Cited Count:5 Percentile:21.81(Physics, Applied)We review recent synchrotron diffraction studies of NaV O
O . The resonant X-ray scattering performed on a monoclinically-split single domain of NaV
. The resonant X-ray scattering performed on a monoclinically-split single domain of NaV O
O shows a critically enhanced contrast between V
shows a critically enhanced contrast between V and V
and V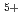 ions. The results has led us to the unequivocal conclusion of the charge-order pattern of low-temperature phase of NaV
ions. The results has led us to the unequivocal conclusion of the charge-order pattern of low-temperature phase of NaV O
O below
below 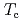 = 35 K. In spite of the possible four types of configuration of the zig-zag-type charge-order patterns in the
= 35 K. In spite of the possible four types of configuration of the zig-zag-type charge-order patterns in the 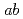 -plane (A, A', B and B'), the stacking sequence along the
-plane (A, A', B and B'), the stacking sequence along the 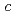 -axis is determined as the AAA'A' type by comparison with model calculations. By assigning the A and A' configurations to Ising spins
-axis is determined as the AAA'A' type by comparison with model calculations. By assigning the A and A' configurations to Ising spins 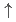 and
and 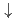 , one can reasonably explain the previously discovered "devil's staircase"-type behavior with respect to the modulation of layer-stacking sequences at high pressures and low temperatures, which clearly resembles the global phase diagram theoretically predicted by the ANNNI model, called "devil's flower". This is the first case that the devil's flower appears in such a charge-ordering system, where charge-order patterns are regarded as Ising spins.
, one can reasonably explain the previously discovered "devil's staircase"-type behavior with respect to the modulation of layer-stacking sequences at high pressures and low temperatures, which clearly resembles the global phase diagram theoretically predicted by the ANNNI model, called "devil's flower". This is the first case that the devil's flower appears in such a charge-ordering system, where charge-order patterns are regarded as Ising spins.
 O
O uniquely determined by resonant X-ray scattering
uniquely determined by resonant X-ray scatteringOwada, Kenji; Fujii, Yasuhiko; Katsuki, Yuya*; Muraoka, Jiro*; Nakao, Hironori*; Murakami, Yoichi; Sawa, Hiroshi*; Ninomiya, Emi*; Isobe, Masahiko*; Ueda, Yutaka*
Physical Review Letters, 94(10), p.106401_1 - 106401_4, 2005/03
Times Cited Count:24 Percentile:72.16(Physics, Multidisciplinary)The present resonant X-ray scattering has been performed on a monoclinically-split single domain of NaV O
O . The observation of a critically enhanced contrast between V
. The observation of a critically enhanced contrast between V and V
and V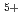 ions has led us to the unequivocal conclusion of the charge-order pattern of low-temperature phase of NaV
ions has led us to the unequivocal conclusion of the charge-order pattern of low-temperature phase of NaV O
O below
below 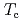 = 35 K. In spite of the possible four types of configuration of the zig-zag-type charge-order patterns in the
= 35 K. In spite of the possible four types of configuration of the zig-zag-type charge-order patterns in the 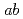 -plane (A, A', B and B'), the stacking sequence along the
-plane (A, A', B and B'), the stacking sequence along the 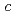 -axis is determined as the AAA'A' type by comparison with model calculations. By assigning the A and A' configurations to Ising spins, one can reasonably explain the previously discovered "devil's staircase"-type behavior with respect to the modulation of layer-stacking sequences at high pressures and low temperatures, which clearly resembles the global phase diagram theoretically predicted by the ANNNI model.
-axis is determined as the AAA'A' type by comparison with model calculations. By assigning the A and A' configurations to Ising spins, one can reasonably explain the previously discovered "devil's staircase"-type behavior with respect to the modulation of layer-stacking sequences at high pressures and low temperatures, which clearly resembles the global phase diagram theoretically predicted by the ANNNI model.

Kodama, Katsuaki; Ikeda, Kazutaka*; Takeda, Hikaru*; Isobe, Masahiko*; Ito, Masayuki*; Ueda, Yutaka*; Shamoto, Shinichi; Otomo, Toshiya*
no journal, ,
no abstracts in English
 by atomic pair distribution function
by atomic pair distribution functionKodama, Katsuaki; Shamoto, Shinichi; Ikeda, Kazutaka*; Otomo, Toshiya*; Takeda, Hikaru*; Isobe, Masahiko*; Ito, Masayuki*; Ueda, Yutaka*
no journal, ,
no abstracts in English

Kodama, Katsuaki; Ikeda, Kazutaka*; Takeda, Hikaru*; Isobe, Masahiko*; Ito, Masayuki*; Ueda, Yutaka*; Shamoto, Shinichi; Otomo, Toshiya*
no journal, ,
no abstracts in English
Suzuki, Eriko; Ogawa, Hiroaki; Nakajima, Kunihisa; Nishioka, Shunichiro; Osaka, Masahiko; Yamashita, Shinichiro; Kurishiba, Ryoko*; Endo, Takashi*; Isobe, Shigehito*; Hashimoto, Naoyuki*
no journal, ,
To elucidate Cs chemisorption behaviour onto stainless steel under LWR severe accident condition, elemental distribution measurement at microlevel was performed by using XPS and TEM. As a result, it was found that Cs-(Fe)-Si-O compounds, which have different composition, could be distributed.
Suzuki, Eriko; Ogawa, Hiroaki; Nakajima, Kunihisa; Nishioka, Shunichiro; Osaka, Masahiko; Yamashita, Shinichiro; Kurishiba, Ryoko*; Endo, Takashi*; Isobe, Shigehito*; Hashimoto, Naoyuki*
no journal, ,
To elucidate Cs chemisorption behaviour onto stainless steel under LWR severe accident condition, elemental distribution measurement at microlevel was performed by using XPS and TEM. As a result, it was found that Cs-(Fe)-Si-O compounds, which have different composition, could be distributed.
Suzuki, Eriko; Nakajima, Kunihisa; Miwa, Shuhei; Osaka, Masahiko; Hashimoto, Naoyuki*; Isobe, Shigehito*
no journal, ,
In order to clarify the revaporization behaviour of cesium (Cs) chemisorbed onto stainless steel under severe accident conditions, the chemical form and distribution of fine Cs chemisorbed compounds were investigated using microscopic analytical methods. It was found that the chemical form and crystal structure differed according to the temperature of Cs chemisorption and depth from the surface. The difference can affect the revaporization rate and revaporized species.
Suzuki, Eriko; Nakajima, Kunihisa; Miwa, Shuhei; Osaka, Masahiko; Hashimoto, Naoyuki*; Isobe, Shigehito*
no journal, ,
In order to improve the cesium (Cs) chemisorption model onto stainless steel (SUS), the chemical forms and distributions of Cs compounds formed inside of the SUS oxide layer at 873-1273 K were evaluated based on the microscopic observation. It was revealed that compositions of Cs compounds varies alonbg depth from the oxide layer surface on SUS, which was tested in 1073-1273 K. In addition, an amorphous phase containing Cs was observed inside of the oxide layer on the SUS tested in 873-973 K. These results suggest that the chemical reactions occurred inside of the SUS oxide layer are different from that in the nearest region of the SUS oxide layer surface.
Suzuki, Eriko; Nakajima, Kunihisa; Miwa, Shuhei; Osaka, Masahiko; Hashimoto, Naoyuki*; Isobe, Shigehito*
no journal, ,
In order to study the cesium (Cs) chemisorption behavior onto stainless steel (SUS) under severe accident conditions, the chemical form and distribution of Cs compounds formed inside of the SUS oxide layer were investigated based on the microscopic observation such as TEM/EDS. As a result, it was revealed that chemical forms of Cs compounds varies along chemisorption temperature and depth from the SUS oxide layer surface.
Suzuki, Eriko; Nakajima, Kunihisa; Miwa, Shuhei; Osaka, Masahiko; Hashimoto, Naoyuki*; Isobe, Shigehito*; Oka, Hiroshi*
no journal, ,
In order to evaluate the properties of chemisorbed cesium (Cs) onto the structural materials in nuclear reactors during severe accidents, temperature dependency of the chemical forms of Cs chemisorbed compounds was investigated based on the compilation of previous studies, the simulation tests of Cs chemisorption onto stainless steel, the acquisition of thermodynamic data and the thermodynamic equilibrium calculations, and the microstructure observation of chemical forms inside the oxide layer. It was revealed that the different Cs chemisorbed compounds were formed depending on the temperature conditions, such as Cs-Fe-O at 873-973 K, Cs-Fe-Si-O at 973-1273 K and Cs-Si-O at 1073-1273 K.
Suzuki, Eriko; Nakajima, Kunihisa; Miwa, Shuhei; Osaka, Masahiko; Hashimoto, Naoyuki*; Isobe, Shigehito*; Oka, Hiroshi*
no journal, ,
In order to evaluate the properties of chemisorbed cesium (Cs) onto the structural materials in nuclear reactors during severe accidents, temperature dependency of the chemical forms of Cs chemisorbed compounds was investigated based on the compilation of previous studies, the simulation tests of Cs chemisorption onto stainless steel, the acquisition of thermodynamic data and the thermodynamic equilibrium calculations, and the microstructure observation of chemical forms inside the oxide layer. It was revealed that the different Cs chemisorbed compounds were formed depending on the temperature conditions, such as Cs-Fe-O at 873-973 K,Cs-Fe-Si-O at 973-1273 K and Cs-Si-O at 1073-1273 K.