Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Mao, W.*; Gong, W.; Kawasaki, Takuro; Gao, S.*; Ito, Tatsuya; Yamashita, Takayuki*; Harjo, S.; Zhao, L.*; Wang, Q.*
Scripta Materialia, 264, p.116726_1 - 116726_6, 2025/07
Times Cited Count:0 Percentile:0.00(Nanoscience & Nanotechnology) neutron diffraction study
neutron diffraction studyIto, Tatsuya; Ogawa, Yuhei*; Gong, W.; Mao, W.*; Kawasaki, Takuro; Okada, Kazuho*; Shibata, Akinobu*; Harjo, S.
Acta Materialia, 287, p.120767_1 - 120767_16, 2025/04
Times Cited Count:1 Percentile:0.00(Materials Science, Multidisciplinary)Ito, Tatsuya; Nagaishi, Ryuji; Kuwano, Ryo*; Godo, Masao*; Yoshida, Yoichi*
Radiation Physics and Chemistry, 226, p.112198_1 - 112198_5, 2025/01
Times Cited Count:0 Percentile:0.00(Chemistry, Physical)In recent years, the use of radiation-resistant resins of polyimide and polyether ether ketone becomes increasing as vessels for irradiation and unsealed radioisotope experiments. However, in our radiolysis experiments, the possibility of interaction between radiolysis products of water and the resin was found, suggesting concerns that the resin may affect reactions in water in radiation fields. To clarify the interaction, dichromate (Cr O
O
 ) reduction and hydrogen peroxide (H
) reduction and hydrogen peroxide (H O
O ) formation in
) formation in  -radiolysis of water were compared with and without the resin. The Cr
-radiolysis of water were compared with and without the resin. The Cr O
O
 reduction amount in aqueous solution with the resin became larger than that without the resin at the same dose, indicating the promotion of Cr
reduction amount in aqueous solution with the resin became larger than that without the resin at the same dose, indicating the promotion of Cr O
O
 reduction by the resin. On the other hand, the H
reduction by the resin. On the other hand, the H O
O formation in pure water with and without an electron scavenger were almost independent of the presence of resin. These suggested the interaction between hydroxyl radical and the resin in contact with water in radiation fields.
formation in pure water with and without an electron scavenger were almost independent of the presence of resin. These suggested the interaction between hydroxyl radical and the resin in contact with water in radiation fields.
Ito, Tatsuya; Xu, S.*; Xu, X.*; Omori, Toshihiro*; Kainuma, Ryosuke*
Shape Memory and Superelasticity, 9 Pages, 2025/00
Yamamoto, Katsuhiro*; Imai, Tatsuya*; Kawai, Atsuki*; Ito, Eri*; Miyazaki, Tsukasa*; Miyata, Noboru*; Yamada, Norifumi*; Seto, Hideki*; Aoki, Hiroyuki
ACS Applied Materials & Interfaces, 16(48), p.66782 - 66791, 2024/11
Times Cited Count:0 Percentile:0.00(Nanoscience & Nanotechnology) neutron diffraction study to elucidate hydrogen effect on the deformation mechanism in Type 310S austenitic stainless steel
neutron diffraction study to elucidate hydrogen effect on the deformation mechanism in Type 310S austenitic stainless steelIto, Tatsuya; Ogawa, Yuhei*; Gong, W.; Mao, W.*; Kawasaki, Takuro; Okada, Kazuho*; Shibata, Akinobu*; Harjo, S.
Proceedings of the 7th International Symposium on Steel Science (ISSS 2024), p.237 - 240, 2024/11
Mao, W.*; Gao, S.*; Gong, W.; Kawasaki, Takuro; Ito, Tatsuya; Harjo, S.; Tsuji, Nobuhiro*
Acta Materialia, 278, p.120233_1 - 120233_13, 2024/10
Times Cited Count:13 Percentile:96.74(Materials Science, Multidisciplinary)Nakajima, Taro*; Saito, Hiraku*; Kobayashi, Naoki*; Kawasaki, Takuro; Nakamura, Tatsuya; Furukawa, Hazuki*; Asai, Shinichiro*; Masuda, Takatsugu*
Journal of the Physical Society of Japan, 93(9), p.091002_1 - 091002_5, 2024/09
Times Cited Count:2 Percentile:64.46(Physics, Multidisciplinary) -dioctylthiodiglycolamic acid; Effect of S donor on metal extraction
-dioctylthiodiglycolamic acid; Effect of S donor on metal extractionShimojo, Kojiro; Fujiwara, Iori*; Saito, Takumi*; Oshima, Tatsuya*
Analytical Sciences, 40(8), p.1429 - 1436, 2024/08
Times Cited Count:0 Percentile:0.00(Chemistry, Analytical)Extraction ability of  -dioctylthiodiglycolamic acid (T-DODGAA), a soft-base sulfur donor ligand with an amide group and a carboxylic acid connected by a thioether chain, for 56 metal ions have been comprehensively investigated and compared with that of N,N-dioctyldiglycolamic acid (DODGAA) with an etheric oxygen atom, a hard-base donor. The p
-dioctylthiodiglycolamic acid (T-DODGAA), a soft-base sulfur donor ligand with an amide group and a carboxylic acid connected by a thioether chain, for 56 metal ions have been comprehensively investigated and compared with that of N,N-dioctyldiglycolamic acid (DODGAA) with an etheric oxygen atom, a hard-base donor. The p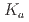 of the thiodiglycolamic acid framework was determined to be 3.71
of the thiodiglycolamic acid framework was determined to be 3.71  0.06 in water (0.1 M LiCl, 25
0.06 in water (0.1 M LiCl, 25 C ) by potentiometric titration, indicating that T-DODGAA is a slightly weaker acid than DODGAA (p
C ) by potentiometric titration, indicating that T-DODGAA is a slightly weaker acid than DODGAA (p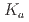 = 3.54
= 3.54  0.03). T-DODGAA can quantitatively extract various metal ions from the 56 metal ions through a proton-exchange reaction. T-DODGAA provided higher extraction performance than DODGAA for Hf(IV), Cr(III), Fe(III), Ni(II), Cu(II), Pd(II), Ag(I), Au(III), Hg(II), Al(III), and Ga(III), especially for soft metal ions. Furthermore, to demonstrate the practical feasibility of T-DODGAA for hydrometallurgy and metal recycling, we performed selective separation tests of rare metal ions such as Sc(III), Ni(II), Co(II), Pd(II), Au(III), In(III), and Ga(II) in metal-mixed extraction systems.
0.03). T-DODGAA can quantitatively extract various metal ions from the 56 metal ions through a proton-exchange reaction. T-DODGAA provided higher extraction performance than DODGAA for Hf(IV), Cr(III), Fe(III), Ni(II), Cu(II), Pd(II), Ag(I), Au(III), Hg(II), Al(III), and Ga(III), especially for soft metal ions. Furthermore, to demonstrate the practical feasibility of T-DODGAA for hydrometallurgy and metal recycling, we performed selective separation tests of rare metal ions such as Sc(III), Ni(II), Co(II), Pd(II), Au(III), In(III), and Ga(II) in metal-mixed extraction systems.
Ito, Tatsuya; Nagaishi, Ryuji; Kuwano, Ryo*
Nuclear Technology, 210(8), p.1427 - 1443, 2024/08
Times Cited Count:0 Percentile:0.00(Nuclear Science & Technology)The retention of hydrogen (H ) bubbles generated by water radiolysis was quantitatively studied in a high-viscous suspension of carbonate slurry consisting of a mixture of suspended solid (SS) of magnesium and calcium precipitates under strongly alkaline conditions, like the radioactive wastes discharged from the coagulation sedimentation (co-precipitation) process at the multinuclide removal equipment in the Fukushima Daiichi Nuclear Power Station. The H
) bubbles generated by water radiolysis was quantitatively studied in a high-viscous suspension of carbonate slurry consisting of a mixture of suspended solid (SS) of magnesium and calcium precipitates under strongly alkaline conditions, like the radioactive wastes discharged from the coagulation sedimentation (co-precipitation) process at the multinuclide removal equipment in the Fukushima Daiichi Nuclear Power Station. The H retention properties were evaluated in two types of carbonate slurry with different hydrophilicity: the hydrophilic "current type" and the hydrophobic "return type". Then, their properties were compared with those in another suspension of clay suspension of bentonite. From the comparison between the amounts of chemical adsorption and H
retention properties were evaluated in two types of carbonate slurry with different hydrophilicity: the hydrophilic "current type" and the hydrophobic "return type". Then, their properties were compared with those in another suspension of clay suspension of bentonite. From the comparison between the amounts of chemical adsorption and H O in the slurry, it was confirmed that H
O in the slurry, it was confirmed that H O molecules must be shared among the SS particles, and this sharing formed the structural viscosity in the slurry, different from that in the clay suspension where electrostatic bonding between the fine clay minerals forms the viscosity. The retention of H
O molecules must be shared among the SS particles, and this sharing formed the structural viscosity in the slurry, different from that in the clay suspension where electrostatic bonding between the fine clay minerals forms the viscosity. The retention of H bubbles in (by) the slurry was evaluated from the difference in the amount of H
bubbles in (by) the slurry was evaluated from the difference in the amount of H observed with and without stirring the slurry after
observed with and without stirring the slurry after  Co
Co  -irradiation. From the comparison of the retention properties of the hydrophilic slurry, the hydrophobic slurry, the clay suspension, and treated water, it was suggested that H2 bubbles were retained not only by the structural viscosity but also by the steric hindrance in the hydrophilic slurry.
-irradiation. From the comparison of the retention properties of the hydrophilic slurry, the hydrophobic slurry, the clay suspension, and treated water, it was suggested that H2 bubbles were retained not only by the structural viscosity but also by the steric hindrance in the hydrophilic slurry.
Hattori, Takanori; Suzuki, Koji*; Miyo, Tatsuya*; Ito, Takayoshi*; Machida, Shinichi*
Nuclear Instruments and Methods in Physics Research A, 1064, p.169448_1 - 169448_9, 2024/07
Times Cited Count:0 Percentile:0.00(Instruments & Instrumentation)The authors regret that the abstract and summary explain that the cup diameter of a standard double-toroidal anvils is 1.5 mm. This is incorrect; the standard diameter is 4.0 mm. The authors would like to apologise for any inconvenience caused.
 Si
Si
Kon, Fusako*; Tabata, Chihiro; Saito, Hiraku*; Nakajima, Taro*; Hidaka, Hiroyuki*; Yanagisawa, Tatsuya*; Amitsuka, Hiroshi*
Journal of the Physical Society of Japan, 93(4), p.044701_1 - 044701_11, 2024/04
Times Cited Count:0 Percentile:0.00(Physics, Multidisciplinary)Osawa, Naoki*; Kim, S.-Y.*; Kubota, Masahiko*; Wu, H.*; Watanabe, So; Ito, Tatsuya; Nagaishi, Ryuji
Nuclear Engineering and Technology, 56(3), p.812 - 818, 2024/03
Times Cited Count:2 Percentile:75.80(Nuclear Science & Technology)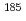 Re in the keV energy region
Re in the keV energy regionKatabuchi, Tatsuya*; Sato, Yaoki*; Takebe, Karin*; Igashira, Masayuki*; Umezawa, Seigo*; Fujioka, Ryo*; Saito, Tatsuhiro*; Iwamoto, Nobuyuki
Journal of Nuclear Science and Technology, 61(2), p.224 - 229, 2024/02
Times Cited Count:0 Percentile:0.00(Nuclear Science & Technology)Imatomi, Daisuke*; Ishikawa, Ryosuke*; Nakata, Akira*; Ito, Tatsuya; Han, K.*; Nagasako, Makoto*; Xu, X.*; Omori, Toshihiro*; Kainuma, Ryosuke*
Journal of Phase Equilibria and Diffusion, 45(1), p.3 - 17, 2024/02
Times Cited Count:1 Percentile:11.66(Chemistry, Physical)Phase equilibria in the Mn-Zn binary system were experimentally determined by chemical composition examination, crystal structure determination, and thermal analysis. Major changes were detected for the  ,
,  , and
, and  phases. The
phases. The  -B2 single-phase region could not be confirmed in the studied system because a disordered body-centered cubic structure, which is identical to the
-B2 single-phase region could not be confirmed in the studied system because a disordered body-centered cubic structure, which is identical to the  Mn phase, was confirmed in a quenched sample from the previously proposed region of
Mn phase, was confirmed in a quenched sample from the previously proposed region of  phase. The
phase. The  phase has been controversial whether the phase is separated into
phase has been controversial whether the phase is separated into  ,
, 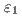 , and
, and 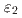 phases or not. By studying a diffusion couple and several alloy compositions, it was established that the
phases or not. By studying a diffusion couple and several alloy compositions, it was established that the  ,
, 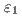 , and
, and 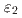 phases are not separate and comprise a single
phases are not separate and comprise a single  phase. Furthermore, the
phase. Furthermore, the  phase is not present in the Zn-rich region of the system because the corresponding invariant reactions were not detected via thermal analysis.
phase is not present in the Zn-rich region of the system because the corresponding invariant reactions were not detected via thermal analysis.
Hattori, Takanori; Suzuki, Koji*; Miyo, Tatsuya*; Ito, Takayoshi*; Machida, Shinichi*
Nuclear Instruments and Methods in Physics Research A, 1059, p.168956_1 - 168956_9, 2024/02
Times Cited Count:2 Percentile:43.92(Instruments & Instrumentation)Radial collimators (RC) with a 0.5 mm gauge size (GS) were specially designed for high-pressure neutron diffraction experiments and their performance and efficacy were investigated. The RCs with nominal GS of 0.75 mm, 1.5 mm, and 3.0 mm effectively exhibited GS of 0.50 mm, 1.07 mm, and 2.78 mm, respectively. The transmissions of all three RCs were almost equivalent. The assessment using a P-E press and a DAC revealed that the anvil scattering was considerably minimized and the sample-to-anvil signal ratio reached values of 0.5 and 2.0 for the PE press and DAC, respectively, when using the 0.5 mm-GS RCs. These results indicate that the 0.5mm-GS RCs have been fabricated as intended and exhibit efficacy for the high-pressure-neutron diffraction experiments, specifically those exceeding 30 GPa. Among those ever manufactured for neutron scattering experiments, the RCs display the smallest GS.
 MnGa metamagnetic shape memory alloy with small energy loss
MnGa metamagnetic shape memory alloy with small energy lossIto, Tatsuya; Xu, X.*; Omori, Toshihiro*; Kainuma, Ryosuke*
Busseiken Dayori, 63(3), p.8 - 10, 2023/10
no abstracts in English
Yamamoto, Kazami; Kinsho, Michikazu; Hayashi, Naoki; Saha, P. K.; Tamura, Fumihiko; Yamamoto, Masanobu; Tani, Norio; Takayanagi, Tomohiro; Kamiya, Junichiro; Shobuda, Yoshihiro; et al.
Journal of Nuclear Science and Technology, 59(9), p.1174 - 1205, 2022/09
Times Cited Count:7 Percentile:73.20(Nuclear Science & Technology)In the Japan Proton Accelerator Research Complex, the purpose of the 3 GeV rapid cycling synchrotron (RCS) is to accelerate a 1 MW, high-intensity proton beam. To achieve beam operation at a repetition rate of 25 Hz at high intensities, the RCS was elaborately designed. After starting the RCS operation, we carefully verified the validity of its design and made certain improvements to establish a reliable operation at higher power as possible. Consequently, we demonstrated beam operation at a high power, namely, 1 MW. We then summarized the design, actual performance, and improvements of the RCS to achieve a 1 MW beam.
 ;
;  SR studies and charge-spin percolation model
SR studies and charge-spin percolation modelSheng, Q.*; Kaneko, Tatsuya*; Yamakawa, Kohtaro*; Guguchia, Z.*; Gong, Z.*; Zhao, G.*; Dai, G.*; Jin, C.*; Guo, S.*; Fu, L.*; et al.
Physical Review Research (Internet), 4(3), p.033172_1 - 033172_14, 2022/09
Ito, Tatsuya; Osugi, Haruka*; Osawa, Naoki*; Takahashi, Tadayuki*; Kim, S.-Y.*; Nagaishi, Ryuji
Analytical Sciences, 38(1), p.91 - 97, 2022/01
Times Cited Count:4 Percentile:21.44(Chemistry, Analytical)A novel ionic liquid (IL) functionalized with thiodiglycol amic acid containing a soft S donor was synthesized for the effective and efficient extraction of platinum group metals (Ru, Rh, and Pd) from aqueous nitric acid solutions, such as high-level radioactive liquid waste (HLLW). The IL allowed a rapid extraction of Pd(II) with an extraction ratio of approximately 100%. The extractions of Ru(III) and Rh(III) by the IL were slower than that of Pd(II), but the rates were accelerated by temperature elevation. The extractions of Ru(III) and Rh(III) at 50 C reached equilibrium within 4 and 8 h, respectively, with the extraction ratios of over 90% without assisting agents or other methods for the extraction system. Furthermore, the IL could extract more than 90% of Ru(III), Rh(III), and Pd(II) from the simulated HLLW within 2 h at 50
C reached equilibrium within 4 and 8 h, respectively, with the extraction ratios of over 90% without assisting agents or other methods for the extraction system. Furthermore, the IL could extract more than 90% of Ru(III), Rh(III), and Pd(II) from the simulated HLLW within 2 h at 50 C.
C.