Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ito, Tatsuya; Nagaishi, Ryuji; Kuwano, Ryo*; Godo, Masao*; Yoshida, Yoichi*
Radiation Physics and Chemistry, 226, p.112198_1 - 112198_5, 2025/01
Times Cited Count:0 Percentile:0.00(Chemistry, Physical)In recent years, the use of radiation-resistant resins of polyimide and polyether ether ketone becomes increasing as vessels for irradiation and unsealed radioisotope experiments. However, in our radiolysis experiments, the possibility of interaction between radiolysis products of water and the resin was found, suggesting concerns that the resin may affect reactions in water in radiation fields. To clarify the interaction, dichromate (Cr O
O
 ) reduction and hydrogen peroxide (H
) reduction and hydrogen peroxide (H O
O ) formation in
) formation in  -radiolysis of water were compared with and without the resin. The Cr
-radiolysis of water were compared with and without the resin. The Cr O
O
 reduction amount in aqueous solution with the resin became larger than that without the resin at the same dose, indicating the promotion of Cr
reduction amount in aqueous solution with the resin became larger than that without the resin at the same dose, indicating the promotion of Cr O
O
 reduction by the resin. On the other hand, the H
reduction by the resin. On the other hand, the H O
O formation in pure water with and without an electron scavenger were almost independent of the presence of resin. These suggested the interaction between hydroxyl radical and the resin in contact with water in radiation fields.
formation in pure water with and without an electron scavenger were almost independent of the presence of resin. These suggested the interaction between hydroxyl radical and the resin in contact with water in radiation fields.
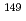 Sm synchrotron-radiation-based M
Sm synchrotron-radiation-based M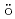 ssbauer spectroscopy of Sm-based heavy fermion compounds
ssbauer spectroscopy of Sm-based heavy fermion compoundsTsutsui, Satoshi; Higashinaka, Ryuji*; Mizumaki, Masaichiro*; Kobayashi, Yoshio*; Nakamura, Jin*; Ito, Takashi; Yoda, Yoshitaka*; Matsuda, Tatsuma*; Aoki, Yuji*; Sato, Hideyuki*
Interactions (Internet), 245(1), p.9_1 - 9_10, 2024/12
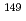 Sm synchrotron-radiation-based M
Sm synchrotron-radiation-based M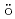 ssbauer and
ssbauer and 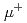 SR studies of Sm
SR studies of Sm Ru
Ru Ge
Ge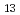
Tsutsui, Satoshi; Ito, Takashi; Nakamura, Jin*; Yoshida, Mio*; Kobayashi, Yoshio*; Yoda, Yoshitaka*; Nakamura, Jumpei*; Koda, Akihiro*; Higashinaka, Ryuji*; Aoki, Dai*; et al.
Interactions (Internet), 245(1), p.55_1 - 55_9, 2024/12
 , AgNbO
, AgNbO , and KNbO
, and KNbO
Yoneda, Yasuhiro; Kobayashi, Toru; Tsuji, Takuya; Matsumura, Daiju; Saito, Yuji; Noguchi, Yuji*
Japanese Journal of Applied Physics, 63(9), p.09SP12_1 - 09SP12_10, 2024/09
Times Cited Count:1 Percentile:35.22(Physics, Applied)NaNbO , AgNbO
, AgNbO , and KNbO
, and KNbO with ABO
with ABO -type perovskite system is known to possess good ferroelectric properties. We directly determined the rattling space of each atom through local structure analysis. This analysis revealed the bonding sites with large fluctuations varied with varying ion size. Experimental evidence including soft X-ray absorption spectroscopy, indicates that the A-site ions are hybridized with oxygen.
-type perovskite system is known to possess good ferroelectric properties. We directly determined the rattling space of each atom through local structure analysis. This analysis revealed the bonding sites with large fluctuations varied with varying ion size. Experimental evidence including soft X-ray absorption spectroscopy, indicates that the A-site ions are hybridized with oxygen.
Ito, Tatsuya; Nagaishi, Ryuji; Kuwano, Ryo*
Nuclear Technology, 210(8), p.1427 - 1443, 2024/08
Times Cited Count:0 Percentile:0.00(Nuclear Science & Technology)The retention of hydrogen (H ) bubbles generated by water radiolysis was quantitatively studied in a high-viscous suspension of carbonate slurry consisting of a mixture of suspended solid (SS) of magnesium and calcium precipitates under strongly alkaline conditions, like the radioactive wastes discharged from the coagulation sedimentation (co-precipitation) process at the multinuclide removal equipment in the Fukushima Daiichi Nuclear Power Station. The H
) bubbles generated by water radiolysis was quantitatively studied in a high-viscous suspension of carbonate slurry consisting of a mixture of suspended solid (SS) of magnesium and calcium precipitates under strongly alkaline conditions, like the radioactive wastes discharged from the coagulation sedimentation (co-precipitation) process at the multinuclide removal equipment in the Fukushima Daiichi Nuclear Power Station. The H retention properties were evaluated in two types of carbonate slurry with different hydrophilicity: the hydrophilic "current type" and the hydrophobic "return type". Then, their properties were compared with those in another suspension of clay suspension of bentonite. From the comparison between the amounts of chemical adsorption and H
retention properties were evaluated in two types of carbonate slurry with different hydrophilicity: the hydrophilic "current type" and the hydrophobic "return type". Then, their properties were compared with those in another suspension of clay suspension of bentonite. From the comparison between the amounts of chemical adsorption and H O in the slurry, it was confirmed that H
O in the slurry, it was confirmed that H O molecules must be shared among the SS particles, and this sharing formed the structural viscosity in the slurry, different from that in the clay suspension where electrostatic bonding between the fine clay minerals forms the viscosity. The retention of H
O molecules must be shared among the SS particles, and this sharing formed the structural viscosity in the slurry, different from that in the clay suspension where electrostatic bonding between the fine clay minerals forms the viscosity. The retention of H bubbles in (by) the slurry was evaluated from the difference in the amount of H
bubbles in (by) the slurry was evaluated from the difference in the amount of H observed with and without stirring the slurry after
observed with and without stirring the slurry after  Co
Co  -irradiation. From the comparison of the retention properties of the hydrophilic slurry, the hydrophobic slurry, the clay suspension, and treated water, it was suggested that H2 bubbles were retained not only by the structural viscosity but also by the steric hindrance in the hydrophilic slurry.
-irradiation. From the comparison of the retention properties of the hydrophilic slurry, the hydrophobic slurry, the clay suspension, and treated water, it was suggested that H2 bubbles were retained not only by the structural viscosity but also by the steric hindrance in the hydrophilic slurry.
Osawa, Naoki*; Kim, S.-Y.*; Kubota, Masahiko*; Wu, H.*; Watanabe, So; Ito, Tatsuya; Nagaishi, Ryuji
Nuclear Engineering and Technology, 56(3), p.812 - 818, 2024/03
Times Cited Count:2 Percentile:75.80(Nuclear Science & Technology) Ge
Ge
Fujiwara, Hidenori*; Nakatani, Yasuhiro*; Aratani, Hidekazu*; Kanai-Nakata, Yuina*; Yamagami, Kohei*; Hamamoto, Satoru*; Kiss, Takayuki*; Sekiyama, Akira*; Tanaka, Arata*; Ebihara, Takao*; et al.
New Physics; Sae Mulli, 73(12), p.1062 - 1066, 2023/12
 Na
Na TiO
TiO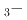 BaTiO
BaTiO solid solutions
solid solutionsYoneda, Yasuhiro; Kobayashi, Toru; Tsuji, Takuya; Matsumura, Daiju; Saito, Yuji; Noguchi, Yuji*
Japanese Journal of Applied Physics, 62(SM), p.SM1006_1 - SM1006_8, 2023/11
Times Cited Count:5 Percentile:47.87(Physics, Applied)Bi Na
Na TiO
TiO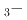 BaTiO
BaTiO (BNT
(BNT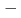 BT) solid solutions have been extensively studied because they exhibit good piezoelectric properties. In addition, a wide variety of phases are observed depending on the BT composition. Soft X-ray absorption spectroscopy and high-energy X-ray diffraction experiments of BNT
BT) solid solutions have been extensively studied because they exhibit good piezoelectric properties. In addition, a wide variety of phases are observed depending on the BT composition. Soft X-ray absorption spectroscopy and high-energy X-ray diffraction experiments of BNT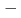 BT solid solutions were performed using synchrotron radiation. From the electronic structure and local structure of BNT
BT solid solutions were performed using synchrotron radiation. From the electronic structure and local structure of BNT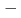 BT solid solution, the substitution effect of BT occurred mainly at the A site, which is the substitution site of Ba. The rhombohedral strain of the TiO
BT solid solution, the substitution effect of BT occurred mainly at the A site, which is the substitution site of Ba. The rhombohedral strain of the TiO octahedron did not change with the change in BT composition, suggesting that the change in the electronic structure at the O-
octahedron did not change with the change in BT composition, suggesting that the change in the electronic structure at the O- absorption edge is due to the change in the hybridization state.
absorption edge is due to the change in the hybridization state.
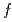 symmetry for anisotropic
symmetry for anisotropic 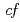 hybridization in the heavy-fermion superconductor CeNi
hybridization in the heavy-fermion superconductor CeNi Ge
Ge
Fujiwara, Hidenori*; Nakatani, Yasuhiro*; Aratani, Hidekazu*; Kanai-Nakata, Yuina*; Yamagami, Kohei*; Hamamoto, Satoru*; Kiss, Takayuki*; Yamasaki, Atsushi*; Higashiya, Atsushi*; Imada, Shin*; et al.
Physical Review B, 108(16), p.165121_1 - 165121_10, 2023/10
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)Ohshima, Hiroyuki; Morishita, Masaki*; Aizawa, Kosuke; Ando, Masanori; Ashida, Takashi; Chikazawa, Yoshitaka; Doda, Norihiro; Enuma, Yasuhiro; Ezure, Toshiki; Fukano, Yoshitaka; et al.
Sodium-cooled Fast Reactors; JSME Series in Thermal and Nuclear Power Generation, Vol.3, 631 Pages, 2022/07
This book is a collection of the past experience of design, construction, and operation of two reactors, the latest knowledge and technology for SFR designs, and the future prospects of SFR development in Japan. It is intended to provide the perspective and the relevant knowledge to enable readers to become more familiar with SFR technology.
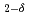 CoGa
CoGa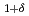 films
filmsKubota, Takahide*; Takano, Daichi*; Kota, Yohei*; Mohanty, S.*; Ito, Keita*; Matsuki, Mitsuhiro*; Hayashida, Masahiro*; Sun, M.*; Takeda, Yukiharu; Saito, Yuji; et al.
Physical Review Materials (Internet), 6(4), p.044405_1 - 044405_12, 2022/04
Times Cited Count:7 Percentile:44.71(Materials Science, Multidisciplinary)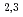 -edge EXAFS and magnetic EXAFS studies on the halfmetallic ferromagnet Co
-edge EXAFS and magnetic EXAFS studies on the halfmetallic ferromagnet Co MnSi
MnSiKogo, Junya*; Fujiwara, Hidenori*; Sekiyama, Akira*; Saito, Yuji; Umetsu, Rie*; Niki, Kaori*
Journal of the Physical Society of Japan, 91(3), p.034702_1 - 034702_12, 2022/03
Times Cited Count:0 Percentile:0.00(Physics, Multidisciplinary)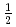 kagome-lattice antiferromagnets Cs
kagome-lattice antiferromagnets Cs Cu
Cu SnF
SnF and Rb
and Rb Cu
Cu SnF
SnF
Saito, Mutsuki*; Takagishi, Ryunosuke*; Kurita, Nubuyuki*; Watanabe, Masari*; Tanaka, Hidekazu*; Nomura, Ryuji*; Fukumoto, Yoshiyuki*; Ikeuchi, Kazuhiko*; Kajimoto, Ryoichi
Physical Review B, 105(6), p.064424_1 - 064424_15, 2022/02
Times Cited Count:7 Percentile:53.46(Materials Science, Multidisciplinary)Ito, Tatsuya; Osugi, Haruka*; Osawa, Naoki*; Takahashi, Tadayuki*; Kim, S.-Y.*; Nagaishi, Ryuji
Analytical Sciences, 38(1), p.91 - 97, 2022/01
Times Cited Count:4 Percentile:21.44(Chemistry, Analytical)A novel ionic liquid (IL) functionalized with thiodiglycol amic acid containing a soft S donor was synthesized for the effective and efficient extraction of platinum group metals (Ru, Rh, and Pd) from aqueous nitric acid solutions, such as high-level radioactive liquid waste (HLLW). The IL allowed a rapid extraction of Pd(II) with an extraction ratio of approximately 100%. The extractions of Ru(III) and Rh(III) by the IL were slower than that of Pd(II), but the rates were accelerated by temperature elevation. The extractions of Ru(III) and Rh(III) at 50 C reached equilibrium within 4 and 8 h, respectively, with the extraction ratios of over 90% without assisting agents or other methods for the extraction system. Furthermore, the IL could extract more than 90% of Ru(III), Rh(III), and Pd(II) from the simulated HLLW within 2 h at 50
C reached equilibrium within 4 and 8 h, respectively, with the extraction ratios of over 90% without assisting agents or other methods for the extraction system. Furthermore, the IL could extract more than 90% of Ru(III), Rh(III), and Pd(II) from the simulated HLLW within 2 h at 50 C.
C.
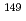 Sm M
Sm M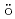 ssbauer and Sm L
ssbauer and Sm L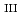 -edge X-ray absorption spectroscopies
-edge X-ray absorption spectroscopiesTsutsui, Satoshi; Higashinaka, Ryuji*; Nakamura, Raito*; Fujiwara, Kosuke*; Nakamura, Jin*; Kobayashi, Yoshio*; Ito, Takashi; Yoda, Yoshitaka*; Kato, Kazuo*; Nitta, Kiyofumi*; et al.
Hyperfine Interactions, 242(1), p.32_1 - 32_10, 2021/12
Times Cited Count:1 Percentile:75.14(Physics, Atomic, Molecular & Chemical)Sakoda, Akihiro; Nomura, Naoki*; Kuroda, Yujiro*; Kono, Takahiko; Naito, Wataru*; Yoshida, Hiroko*
Journal of Radiological Protection, 41(4), p.1258 - 1287, 2021/12
Times Cited Count:1 Percentile:6.95(Environmental Sciences)Following the Fukushima Daiichi Nuclear Power Plant accident, many radiation experts directly experienced a vast gap between ideal and real public understanding (PU) of radiation in risk communication. Therefore, this study collated and reviewed information about PU activities for radiation and its risk that six Japanese academic societies - which seemed to be socially neutral expert communities - related to radiation and radiation risk conducted before and after the accident. Activities these radiation-related societies provided to general public were discussed from the following perspectives: (1) difficulties in two-way communication due to resources, motivation, public interest and concerns; (2) balance between academic research and PU activities; (3) academic societies' building trust with the public whilst ensuring member experts' neutrality and independence; (4) discussions among academic societies to prepare for public engagement. We hope that this paper encourages experts and academic societies in radiation protection to hold more national and international discussions about their roles in public communication and outreach.
Yoshida, Hiroko*; Kuroda, Yujiro*; Kono, Takahiko; Naito, Wataru*; Sakoda, Akihiro
Journal of Radiation Protection and Research, 46(3), p.134 - 142, 2021/09
The Japan Health Physics Society established a task group on "Public Understanding after the Fukushima Daiichi Nuclear Power Plant Accident" in the 2018-2019 fiscal year. This task group collected and analyzed various activities that had been made for promotion of public understanding since the Fukushima accident, and then discussed some issues such as expert's roles. This paper outlines a panel session for this task group held at the 53rd Annual Meeting of the Japanese Health Physics Society (Online). This session consisted of (1) reporting what the task group achieved, (2) having comments by two designated experts in the fields of sociology and ethics, (3) making a panel discussion with three representatives from the task group and the two designated speakers, and (4) summarizing this session by a rapporteur.
Fujiwara, Hidenori*; Umetsu, Rie*; Kuroda, Fumiaki*; Miyawaki, Jun*; Kashiuchi, Toshiyuki*; Nishimoto, Kohei*; Nagai, Kodai*; Sekiyama, Akira*; Irizawa, Akinori*; Takeda, Yukiharu; et al.
Scientific Reports (Internet), 11(1), p.18654_1 - 18654_9, 2021/09
Times Cited Count:0 Percentile:0.00(Multidisciplinary Sciences) VAl/Fe layered films showing exchange bias
VAl/Fe layered films showing exchange biasKubota, Takahide*; Shimada, Yusuke*; Tsuchiya, Tomoki*; Yoshikawa, Tomoki*; Ito, Keita*; Takeda, Yukiharu; Saito, Yuji; Konno, Toyohiko*; Kimura, Akio*; Takanashi, Koki*
Nanomaterials (Internet), 11(7), p.1723_1 - 1723_11, 2021/07
Times Cited Count:2 Percentile:10.46(Chemistry, Multidisciplinary)Sasaki, Yuji; Morita, Keisuke; Kitatsuji, Yoshihiro; Ito, Keisuke*; Yoshizuka, Kazuharu*
Solvent Extraction Research and Development, Japan, 28(2), p.121 - 131, 2021/00
Times Cited Count:2 Percentile:8.76(Chemistry, Multidisciplinary)High concentration of Cs is present in high-level radioactive waste. It is well-known that Cs is an alkali element and difficult to extract completely into an organic phase. Crown ether compounds are widely available for Cs extractants; DtBuDB18C6 (di- -butyl-dibenzo-18crown6), was used in this study. Organic solvents used for the industrial applications, such as
-butyl-dibenzo-18crown6), was used in this study. Organic solvents used for the industrial applications, such as  -dodecane and 1-octanol, have low solubility concerning the compound; other solvents were employed and tested. In this study, ketone-, ether-, and ester-type solvents showed high solubility for DtBuDB18C6 and DtBuDB18C6, when dissolved in ketones and alcohols, exhibited relatively high Cs distribution ratios (
-dodecane and 1-octanol, have low solubility concerning the compound; other solvents were employed and tested. In this study, ketone-, ether-, and ester-type solvents showed high solubility for DtBuDB18C6 and DtBuDB18C6, when dissolved in ketones and alcohols, exhibited relatively high Cs distribution ratios ( (Cs)), closely to 10.
(Cs)), closely to 10.