Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hamamoto, Takafumi*; Koike, Ayaka*; Ishidera, Takamitsu; Iwata, Hajime; Fukatsu, Yuta; Taneichi, Yayoi
NUMO-TR-24-03, p.85 - 86, 2024/10
no abstracts in English
Matsubara, Ryuta*; Ueno, Fuga*; Iwata, Hajime; Inagaki, Yaohiro*; Okubo, Takahiro*
NUMO-TR-24-03, p.55 - 61, 2024/10
no abstracts in English
Kimuro, Shingo; Taneichi, Yayoi; Iwata, Hajime; Ishidera, Takamitsu; Kitamura, Akira; Tachi, Yukio; Tanaka, Takeru*; Hirano, Kana*; Hieda, Manami*; Miyabe, Shunsuke*; et al.
Journal of Solution Chemistry, 53(6), p.854 - 868, 2024/06
Times Cited Count:0 Percentile:0.00(Chemistry, Physical)Miyazaki, Kanako*; Takehara, Masato*; Minomo, Kenta*; Horie, Kenji*; Takehara, Mami*; Yamasaki, Shinya*; Saito, Takumi*; Onuki, Toshihiko*; Takano, Masahide; Shiotsu, Hiroyuki; et al.
Journal of Hazardous Materials, 470(15), p.134104_1 - 134104_11, 2024/05
Times Cited Count:1 Percentile:31.14(Engineering, Environmental)Matsubara, Ryuta*; Takubo, Yusaku*; Iwata, Hajime; Inagaki, Yaohiro*; Okubo, Takahiro*
NUMO-TR-24-01, p.104 - 108, 2024/05
no abstracts in English
Kitazato, Kohei*; Milliken, R. E.*; Iwata, Takahiro*; Abe, Masanao*; Otake, Makiko*; Matsuura, Shuji*; Takagi, Yasuhiko*; Nakamura, Tomoki*; Hiroi, Takahiro*; Matsuoka, Moe*; et al.
Nature Astronomy (Internet), 5(3), p.246 - 250, 2021/03
Times Cited Count:60 Percentile:96.18(Astronomy & Astrophysics)Here we report observations of Ryugu's subsurface material by the Near-Infrared Spectrometer (NIRS3) on the Hayabusa2 spacecraft. Reflectance spectra of excavated material exhibit a hydroxyl (OH) absorption feature that is slightly stronger and peak-shifted compared with that observed for the surface, indicating that space weathering and/or radiative heating have caused subtle spectral changes in the uppermost surface. However, the strength and shape of the OH feature still suggests that the subsurface material experienced heating above 300  C, similar to the surface. In contrast, thermophysical modeling indicates that radiative heating does not increase the temperature above 200
C, similar to the surface. In contrast, thermophysical modeling indicates that radiative heating does not increase the temperature above 200  C at the estimated excavation depth of 1 m, even if the semimajor axis is reduced to 0.344 au. This supports the hypothesis that primary thermal alteration occurred due to radiogenic and/or impact heating on Ryugu's parent body.
C at the estimated excavation depth of 1 m, even if the semimajor axis is reduced to 0.344 au. This supports the hypothesis that primary thermal alteration occurred due to radiogenic and/or impact heating on Ryugu's parent body.
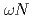 scattering length from
scattering length from 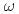 photoproduction on the proton near the reaction threshold
photoproduction on the proton near the reaction thresholdIshikawa, Takatsugu*; Fujimura, Hisako*; Fukasawa, Hiroshi*; Hashimoto, Ryo*; He, Q.*; Honda, Yuki*; Hosaka, Atsushi; Iwata, Takahiro*; Kaida, Shun*; Kasagi, Jirota*; et al.
Physical Review C, 101(5), p.052201_1 - 052201_6, 2020/05
Times Cited Count:4 Percentile:36.67(Physics, Nuclear)Kaneko, Makoto*; Iwata, Hajime; Shiotsu, Hiroyuki; Masaki, Shota*; Kawamoto, Yuji*; Yamasaki, Shinya*; Nakamatsu, Yuki*; Imoto, Jumpei*; Furuki, Genki*; Ochiai, Asumi*; et al.
Frontiers in Energy Research (Internet), 3, p.37_1 - 37_10, 2015/09
The mobility of the aggregates of submicron-sized sheet aluminosilicate in the surface environment is a key factor controlling the current Cs migration in Fukushima.
Doi, Reisuke; Iwata, Hajime; Kitamura, Akira
JAEA-Review 2014-014, 27 Pages, 2014/05
The solubility method is one of the most powerful tools to obtain reliable thermodynamic data for (1) solubility products of discrete solids and double salts, (2) complexation constants for various ligands, (3) development of data in a wide range of pH values, (4) evaluation of data for metals that form very insoluble solids (e.g. tetravalent actinides), (5) determining solubility-controlling solids in different types of wastes and (6) elevated temperatures for redox sensitive systems. This document is focused on describing various aspects of obtaining thermodynamic data using the solubility method. This manuscript deals with various aspects of conducting solubility studies, including selecting the study topic, modeling to define important variables, selecting the range of variables and experimental parameters, anticipating results, general equipment requirements, conducting experiments, and interpreting experimental data.
 (
(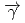 ,
,
 )
)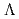 and
and  (
(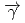 ,
,
 )
)
 reactions for
reactions for 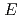
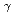 =1.5-2.4 GeV
=1.5-2.4 GeVZegers, R. G. T.*; Sumihama, Mizuki*; Ahn, D. S.*; Ahn, J. K.*; Akimune, Hidetoshi*; Asano, Yoshihiro; Chang, W. C.*; Dat , S.*; Ejiri, Hiroyasu*; Fujimura, Hisako*; et al.
, S.*; Ejiri, Hiroyasu*; Fujimura, Hisako*; et al.
Physical Review Letters, 91(9), p.092001_1 - 092001_4, 2003/08
Times Cited Count:128 Percentile:94.59(Physics, Multidisciplinary)no abstracts in English
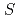 = +1 Baryon resonance in photoproduction from the neutron
= +1 Baryon resonance in photoproduction from the neutronNakano, Takashi*; Ahn, D. S.*; Ahn, J. K.*; Akimune, Hidetoshi*; Asano, Yoshihiro; Chang, W. C.*; Date, S.*; Ejiri, Hiroyasu*; Fujimura, Hisako*; Fujiwara, Mamoru; et al.
Physical Review Letters, 91(1), p.012002_1 - 012002_4, 2003/07
Times Cited Count:1023 Percentile:99.84(Physics, Multidisciplinary)no abstracts in English
Ueno, Fumiyoshi; Kano, Shigeki; Fujita, Mitsutane*; Kurihara, Yutaka*; Nakajima, Hajime*; Yokoyama, Norio*; Iwata, Shuichi*
Journal of Nuclear Science and Technology, 31(12), p.1314 - 1324, 1994/00
None
Fujita, Mitsutane*; ; Nakajima, Hajime; ; Ueno, Fumiyoshi*; Kano, Shigeki*; Iwata, Shuichi*
Computer Aided Innovation of New Materials,II,Pt. 1, p.81 - 84, 1993/00
no abstracts in English
Fujita, Mitsutane*; Nakajima, Hajime; Ueno, Fumiyoshi*; Iwata, Shuichi*
Genshiryoku Kogyo, 39(10), p.53 - 57, 1993/00
no abstracts in English
Fujita, Mitsutane*; ; Nakajima, Hajime; ; Ueno, Fumiyoshi*; ; Iwata, Shuichi*
Proc. of the 4th Int. Symp. on Advanced Nuclear Energy Research (JAERI-CONF 1/JAERI-M 92-207), p.402 - 409, 1992/12
no abstracts in English
Nakajima, Hajime; ; ; Ueno, Fumiyoshi*; Fujita, Mitsutane*; ; Iwata, Shuichi*
Journal of Nuclear Materials, 191-194, p.1046 - 1050, 1992/00
Times Cited Count:4 Percentile:41.57(Materials Science, Multidisciplinary)no abstracts in English
Fujita, Mitsutane*; ; Nakajima, Hajime; ; Ueno, Fumiyoshi*; Kano, Shigeki*; Iwata, Shuichi*
Proc. of the Int. Symp. on Material Chemistry in Nuclear Environment, p.601 - 611, 1992/00
no abstracts in English
Fujita, Mitsutane*; Nakajima, Hajime; ; Iwata, Shuichi*
Computer Aided Innovation of New Materials, p.25 - 28, 1991/00
no abstracts in English
Fujita, Mitsutane*; ; Nakajima, Hajime; ; ; Ueno, Fumiyoshi*; Iwata, Shuichi*
Genshiro Zairyo Dai-122 Iinkai Heisei-2-Nendo Dai-5-Kai Iinkai Shiryo (Nihon Gakujutsu Shinkokai), p.7 - 14, 1991/00
no abstracts in English
Iwata, Hajime; Mitsui, Seiichiro; Sekine, Nobuyuki*
no journal, ,
In order to measure Fe-silicates precipitated under geological disposal conditions, a static leaching test has been conducted of simulated vitrified waste in 0.01 M FeCl solution with a surface area to volume ratio = 10 m
solution with a surface area to volume ratio = 10 m at 90
at 90 C under a N
C under a N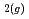 atmosphere. Obtained results indicate that Fe bearing minerals change from green rust to Fe-rich serpentine over time. Such mineral transitions are important as they may have an effect on the alteration behavior of glasses used for geological disposal of HLW.
atmosphere. Obtained results indicate that Fe bearing minerals change from green rust to Fe-rich serpentine over time. Such mineral transitions are important as they may have an effect on the alteration behavior of glasses used for geological disposal of HLW.