Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ito, Takachika*; Suzuki, Shoichi*; Kanaji, Sachiko*; Shiraishi, Hiroshi*; Ota, Shoichiro*; Arima, Kazuhiko*; Tanaka, Go*; Tamada, Taro; Honjo, Eijiro*; Garcia, K. C.*; et al.
Journal of Biological Chemistry, 284(36), p.24289 - 24296, 2009/09
Times Cited Count:23 Percentile:45.4(Biochemistry & Molecular Biology)Both IL-4 and IL-13 can bind to the shared receptor composed of the IL-4 receptor  chain and the IL-13 receptor
chain and the IL-13 receptor  -1 chain (IL-13R
-1 chain (IL-13R 1); however, the assembly mechanisms of these ligands to the receptor is different, enabling the principal functions of these ligands to be different. We have previously shown that the N-terminal Ig-like domain in IL-13R
1); however, the assembly mechanisms of these ligands to the receptor is different, enabling the principal functions of these ligands to be different. We have previously shown that the N-terminal Ig-like domain in IL-13R 1, called the D1 domain, is the specific and critical binding unit for IL-13. However, it has still remained obscure which the amino acid has specific binding capacity to IL-13 and why the D1 domain acts as the binding site for IL-13, but not IL-4. To address these questions, in this study, we performed the mutational analyses for the D1 domain, combining the structural data to identify the amino acids critical for binding to IL-13. Mutations of Lys76, Lys77, or Ile78 in c' strand in which the crystal structure showed interact with IL-13 and those of Trp65 and Ala79 adjacent to the interacting site, resulted in significant impairment of IL-13 binding, demonstrating that these amino acids generate the binding site. Furthermore, mutations of Val35, Leu38, or Val42 at N-terminal
1, called the D1 domain, is the specific and critical binding unit for IL-13. However, it has still remained obscure which the amino acid has specific binding capacity to IL-13 and why the D1 domain acts as the binding site for IL-13, but not IL-4. To address these questions, in this study, we performed the mutational analyses for the D1 domain, combining the structural data to identify the amino acids critical for binding to IL-13. Mutations of Lys76, Lys77, or Ile78 in c' strand in which the crystal structure showed interact with IL-13 and those of Trp65 and Ala79 adjacent to the interacting site, resulted in significant impairment of IL-13 binding, demonstrating that these amino acids generate the binding site. Furthermore, mutations of Val35, Leu38, or Val42 at N-terminal  -strand also resulted in loss of IL-13 binding, probably from decrease structural stability. None of the mutations employed here affected IL-4 binding. These results demonstrate that the hydrophobic patch composed of Lys76, Lys77, and Ile78 is the IL-13 recognition site and solidify our understanding that the differential requirements of the D1 domain in IL-13R
-strand also resulted in loss of IL-13 binding, probably from decrease structural stability. None of the mutations employed here affected IL-4 binding. These results demonstrate that the hydrophobic patch composed of Lys76, Lys77, and Ile78 is the IL-13 recognition site and solidify our understanding that the differential requirements of the D1 domain in IL-13R 1 allows the shared receptor to respond differentially to IL-4 and IL-13.
1 allows the shared receptor to respond differentially to IL-4 and IL-13.
 chain and interleukin-13 receptor
chain and interleukin-13 receptor  1 chain by a silkworm-baculovirus system
1 chain by a silkworm-baculovirus systemHonjo, Eijiro; Shoyama, Yoshinari; Tamada, Taro; Shigematsu, Hideki*; Hatanaka, Takaaki*; Kanaji, Sachiko*; Arima, Kazuhiko*; Ito, Yuji*; Izuhara, Kenji*; Kuroki, Ryota
Protein Expression and Purification, 60(1), p.25 - 30, 2008/07
Times Cited Count:13 Percentile:33.69(Biochemical Research Methods)The receptor binding to Interleukin (IL)-13 is composed of the IL-13 receptor  1 chain (IL-13R
1 chain (IL-13R  1) and the IL-4 receptor
1) and the IL-4 receptor  chain (IL-4R
chain (IL-4R  ). In order to investigate the interaction of IL-13 with IL-13R
). In order to investigate the interaction of IL-13 with IL-13R  1 and IL-4R
1 and IL-4R  , the DNA fragments coding the extracellular regions of human IL-13R
, the DNA fragments coding the extracellular regions of human IL-13R  1 and the IL-4R
1 and the IL-4R  were fused with mouse Fc and expressed by a silkworm-baculovirus system. The expressed receptors were successfully purified by affinity chromatography using protein A, and the Fc region was removed by thrombin digestion. Size exclusion chromatography and SPR analysis revealed that mixture of IL-13 and IL-13R
were fused with mouse Fc and expressed by a silkworm-baculovirus system. The expressed receptors were successfully purified by affinity chromatography using protein A, and the Fc region was removed by thrombin digestion. Size exclusion chromatography and SPR analysis revealed that mixture of IL-13 and IL-13R 1 showed predominant affinity to IL-4R
1 showed predominant affinity to IL-4R , although neither detectable affinity of IL-13 nor IL-13R
, although neither detectable affinity of IL-13 nor IL-13R 1 was observed against IL-4R
1 was observed against IL-4R . Combining these data with the moderate affinity of IL-13 to IL-13R
. Combining these data with the moderate affinity of IL-13 to IL-13R 1, this indicates that IL-13 first binds to IL-13R
1, this indicates that IL-13 first binds to IL-13R 1 and recruits consequently to IL-4R.
1 and recruits consequently to IL-4R.
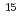 N NMR relaxation measurements
N NMR relaxation measurementsYoshida, Yuichiro*; Okuri, Takatoshi*; Takeda, Chika*; Kuroki, Ryota; Izuhara, Kenji*; Imoto, Taiji*; Ueda, Tadashi*
Biochemical and Biophysical Research Communications, 358(1), p.292 - 297, 2007/06
Times Cited Count:4 Percentile:9.9(Biochemistry & Molecular Biology)The single nucleotide polymorphism interleukin-13 (IL-13) R110Q is associated with severe bronchial asthma because its lower affinity leads to the augmentation of local IL-13 concentration, resulting in an increase in the signal transduction via IL-13R. Since the mutation site does not directly bind to IL-13 R 2, we carried out NMR relaxation analyses of the wild-type IL-13 and IL-13 R110Q in order to examine whether the R110Q mutation affects the internal motions in IL-13 molecules. The results showed that the internal motion in the micro- to millisecond time scale on helix D, which is suggested to be important for the interaction between IL-13 and IL-13R
2, we carried out NMR relaxation analyses of the wild-type IL-13 and IL-13 R110Q in order to examine whether the R110Q mutation affects the internal motions in IL-13 molecules. The results showed that the internal motion in the micro- to millisecond time scale on helix D, which is suggested to be important for the interaction between IL-13 and IL-13R 2, is increased in IL-13-R110Q compared with that in the wild-type IL-13. It therefore appears that the difference in the internal motions on helix D between the wild-type IL-13 and IL-13-R110Q may be involved in their affinity differences with IL-13R
2, is increased in IL-13-R110Q compared with that in the wild-type IL-13. It therefore appears that the difference in the internal motions on helix D between the wild-type IL-13 and IL-13-R110Q may be involved in their affinity differences with IL-13R 2.
2.
 1 chain and its interaction with its ligand
1 chain and its interaction with its ligandKuroki, Ryota; Honjo, Eijiro; Tamada, Taro; Arima, Kazuhiko*; Izuhara, Kenji*
no journal, ,
Interleukin-13 (IL-13) interacts with two types of receptors, the IL-13R 1 chain (IL-13R
1 chain (IL-13R 1) and the IL-4R
1) and the IL-4R chain (IL-4R
chain (IL-4R ), that transduce the IL-13 signals. In order to investigate the interaction of IL-13 with IL-13R
), that transduce the IL-13 signals. In order to investigate the interaction of IL-13 with IL-13R 1 and IL-4R
1 and IL-4R , the genes coding the extracellular regions of human IL-13R
, the genes coding the extracellular regions of human IL-13R 1 and the IL-4R
1 and the IL-4R containing cytokine receptor homologous region (CRH), were fused with human Fc, and expressed by silk worm. After further purification with anion-exchange chromatography, the receptors were used to investigate the ligand-receptor interaction. The size exclusion chromatography showed that association between IL-13 and IL-13Ra1 could not be observed in this condition whereas the clear association of IL-13 and IL-13Ra1 was observed after adding IL-4R
containing cytokine receptor homologous region (CRH), were fused with human Fc, and expressed by silk worm. After further purification with anion-exchange chromatography, the receptors were used to investigate the ligand-receptor interaction. The size exclusion chromatography showed that association between IL-13 and IL-13Ra1 could not be observed in this condition whereas the clear association of IL-13 and IL-13Ra1 was observed after adding IL-4R . It indicates that the association between IL-13 and IL-4R
. It indicates that the association between IL-13 and IL-4R increase the IL-13 affinity to IL-13R
increase the IL-13 affinity to IL-13R 1.
1.
 1 chain and IL-4 receptor
1 chain and IL-4 receptor 
Honjo, Eijiro; Shoyama, Yoshinari; Tamada, Taro; Arima, Kazuhiko*; Kanaji, Sachiko*; Izuhara, Kenji*; Kuroki, Ryota
no journal, ,
The receptor binding to Interleukin (IL)-13 is composed of the IL-13 receptor  1 chain (IL-13R
1 chain (IL-13R 1) and the IL-4 receptor
1) and the IL-4 receptor  chain (IL-4R
chain (IL-4R ). In order to investigate the interaction of IL-13 with IL-13R
). In order to investigate the interaction of IL-13 with IL-13R 1 and IL-4R
1 and IL-4R , the DNA fragments coding the extracellular regions of human IL-13R
, the DNA fragments coding the extracellular regions of human IL-13R 1 and the IL-4R
1 and the IL-4R , containing a cytokine receptor homologous region, were fused with human Fc and expressed by silkworm-baculovirus system. Size exclusion chromatography did not reveal association between IL-13 and IL-13R
, containing a cytokine receptor homologous region, were fused with human Fc and expressed by silkworm-baculovirus system. Size exclusion chromatography did not reveal association between IL-13 and IL-13R 1 under these conditions, but the clear association of IL-13 and IL-13R
1 under these conditions, but the clear association of IL-13 and IL-13R 1 was observed after adding IL-4R
1 was observed after adding IL-4R . This indicates that both extracellular regions of IL-13R
. This indicates that both extracellular regions of IL-13R 1 and IL-4R
1 and IL-4R have functions, and the association between IL-13 and IL-4R
have functions, and the association between IL-13 and IL-4R increases the IL-13 affinity to IL-13R
increases the IL-13 affinity to IL-13R 1.
1.
 2 chain (IL-13R
2 chain (IL-13R 2)
2)Matsumoto, Fumiko; Tamada, Taro; Honjo, Eijiro*; Ota, Shoichiro*; Izuhara, Kenji*; Kuroki, Ryota
no journal, ,
The allergic disease has dramatically increased in recent years. Interleukin-13 (IL-13) is a key cytokine critical to the development of T-cell-mediated humoral immune responses which are associated with allergy and asthma. IL-13 possesses two types of receptor: the heterodimer, composed of IL-13R 1 and IL-4R
1 and IL-4R , transducing the IL-13 signals; and the IL-13R
, transducing the IL-13 signals; and the IL-13R 2, acting as a nonsignaling "decoy" receptor. Although the extracellular region of IL-13R
2, acting as a nonsignaling "decoy" receptor. Although the extracellular region of IL-13R 1 and IL-13R
1 and IL-13R 2 are composed of a common structure of the class 1 cytokine receptor superfamily and they have similar amino acid sequence, the affinity of IL-13R
2 are composed of a common structure of the class 1 cytokine receptor superfamily and they have similar amino acid sequence, the affinity of IL-13R 2 with IL-13 is more than ten-fold higher than that of IL-13R
2 with IL-13 is more than ten-fold higher than that of IL-13R 1. In order to investigate the reason for this higher ligand affinity to IL-13R
1. In order to investigate the reason for this higher ligand affinity to IL-13R 2, extra cellular region of IL-13R
2, extra cellular region of IL-13R 2 was expressed by silkworm-baculovirus expression system after fusing the DNA cording the extracellular region of human IL-13R
2 was expressed by silkworm-baculovirus expression system after fusing the DNA cording the extracellular region of human IL-13R 2 with the Fc derived from mouse IgG2a. The expressed fusion protein IL-13R
2 with the Fc derived from mouse IgG2a. The expressed fusion protein IL-13R 2-Fc was purified by a protein G column, and the affinity to IL-13 was confirmed by gel filtration followed by light scattering analysis. After Fc region was removed by thrombin digestion, the resulting extra cellular region of IL-13R
2-Fc was purified by a protein G column, and the affinity to IL-13 was confirmed by gel filtration followed by light scattering analysis. After Fc region was removed by thrombin digestion, the resulting extra cellular region of IL-13R 2 receptor was confirmed to retain strong affinity to IL-13 with 1:1 ratio.
2 receptor was confirmed to retain strong affinity to IL-13 with 1:1 ratio.
 2 chain
2 chainMatsumoto, Fumiko; Hatanaka, Takaaki*; Tamada, Taro; Honjo, Eijiro*; Ota, Shoichiro*; Ito, Yuji*; Izuhara, Kenji*; Kuroki, Ryota
no journal, ,
Interleukin-13 (IL-13) is a key cytokine critical to the development of T-cell-mediated humoral immune responses which are associated with allergy and asthma. IL-13 possesses two types of receptor: the heterodimer, composed of IL-13R 1 and IL-4R
1 and IL-4R , transducing the IL-13 signals; and the IL-13R
, transducing the IL-13 signals; and the IL-13R 2, it has high affinity rather than IL-13R
2, it has high affinity rather than IL-13R 1 against IL-13, acting as a nonsignaling "decoy" receptor. In order to investigate the inhibition mechanism of IL-13 signal by IL-13R
1 against IL-13, acting as a nonsignaling "decoy" receptor. In order to investigate the inhibition mechanism of IL-13 signal by IL-13R 2, extra cellular region of IL-13R
2, extra cellular region of IL-13R 2 was expressed by silkworm-baculovirus expression system after fusing the DNA cording the extracellular region of human IL-13R
2 was expressed by silkworm-baculovirus expression system after fusing the DNA cording the extracellular region of human IL-13R 2 with the Fc derived from mouse IgG2a. The expressed fusion protein (IL-13R
2 with the Fc derived from mouse IgG2a. The expressed fusion protein (IL-13R 2)2-Fc was purified by a protein A column followed by an ion exchange column. The affinities of the ligand complexes, IL-13/IL-13R
2)2-Fc was purified by a protein A column followed by an ion exchange column. The affinities of the ligand complexes, IL-13/IL-13R 1 and IL-13/IL-13R
1 and IL-13/IL-13R 2 to (IL-4R
2 to (IL-4R )2-Fc were investigated to explore the inactivation mechanism of the IL-13R
)2-Fc were investigated to explore the inactivation mechanism of the IL-13R 1 signal with IL-13R
1 signal with IL-13R 2 by surface plasmon resonance analysis. IL-13/IL-13R
2 by surface plasmon resonance analysis. IL-13/IL-13R 1 complex has an affinity (KD=1.20
1 complex has an affinity (KD=1.20 10
10 M) with (IL-4Ra)2-Fc, whereas the IL-13/IL-13R
M) with (IL-4Ra)2-Fc, whereas the IL-13/IL-13R 2 complex had no affinity with (IL-4R
2 complex had no affinity with (IL-4R )2-Fc. This observation suggests that IL-13R
)2-Fc. This observation suggests that IL-13R 2 does not inactivate the IL-13R
2 does not inactivate the IL-13R 1 through formation of a ternary complex with IL-13 and IL-4R
1 through formation of a ternary complex with IL-13 and IL-4R , but removes the IL-13 with its higher affinity to IL-13 without forming a ternary complex.
, but removes the IL-13 with its higher affinity to IL-13 without forming a ternary complex.