Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Toyoshima, Atsushi; Kanda, Akimitsu*; Ikeda, Takumi*; Yoshimura, Takashi*; Shinohara, Atsushi*; Yano, Shinya*; Komori, Yukiko*; Haba, Hiromitsu*
no journal, ,
Astatine (At) is reported to be bound in a few oxidation states in aqueous solutions. However, their valencies and chemical species are experimentally not identified. In this study, we studied redox behavior of At using redox agents and using a flow electrolytic column.  At was produced in the
At was produced in the 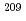 Bi(
Bi( , 2
, 2 )
) At reaction and was then separated from the target by a distillation method. Then, redox of At was carried out in 1.0 M HClO
At reaction and was then separated from the target by a distillation method. Then, redox of At was carried out in 1.0 M HClO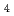 using redox agents or by an electrolysis. After the redox, solvent extraction with HDEHP was performed to identify the redox reactions. Using the redox agents, extraction yields of At were different between the conditions with oxidizing or reducing agents and without redox agents. This means that redox reactions of At can be identified under the present extraction conditions. On the other hand, by the electrolysis, extraction yields of At were almost constant against the variation of applied potential. This is possibly due to the restoration to the original oxidation state after the electrolysis. In the future, we will perform flow electrolytic column chromatography available for simultaneous electrolysis and separation.
using redox agents or by an electrolysis. After the redox, solvent extraction with HDEHP was performed to identify the redox reactions. Using the redox agents, extraction yields of At were different between the conditions with oxidizing or reducing agents and without redox agents. This means that redox reactions of At can be identified under the present extraction conditions. On the other hand, by the electrolysis, extraction yields of At were almost constant against the variation of applied potential. This is possibly due to the restoration to the original oxidation state after the electrolysis. In the future, we will perform flow electrolytic column chromatography available for simultaneous electrolysis and separation.