Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Fujita, Yoshitaka; Niizeki, Tomotake*; Fukumitsu, Nobuyoshi*; Ariga, Katsuhiko*; Yamauchi, Yusuke*; Malgras, V.*; Kaneti, Y. V.*; Liu, C.-H.*; Hatano, Kentaro*; Suematsu, Hisayuki*; et al.
Bulletin of the Chemical Society of Japan, 95(1), p.129 - 137, 2022/01
Times Cited Count:8 Percentile:76.16(Chemistry, Multidisciplinary)In this work, the mechanisms responsible for the adsorption of molybdate ions on alumina are investigated using in-depth surface analyses carried out on alumina specimens immersed in solutions containing different molybdate ions at different pH values. The obtained results reveal that when alumina is immersed in an acidic solution containing molybdate ions, the hydroxyl groups present on the surface are removed to generate positively charged sites, and molybdate ions (MoO
 or AlMo
or AlMo O
O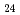 H
H
 ) are adsorbed by electrostatic interaction. Alumina dissolves slightly in an acidic solution to form AlMo
) are adsorbed by electrostatic interaction. Alumina dissolves slightly in an acidic solution to form AlMo O
O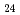 H
H
 , which is more easily desorbed than MoO
, which is more easily desorbed than MoO
 . Furthermore, the enhancement in the Mo adsorption or desorption property may be achieved by enriching the surface of the alumina adsorbent with many -OH groups and optimizing Mo solution to adsorb molybdate ions on alumina as MoO
. Furthermore, the enhancement in the Mo adsorption or desorption property may be achieved by enriching the surface of the alumina adsorbent with many -OH groups and optimizing Mo solution to adsorb molybdate ions on alumina as MoO
 ions. These findings will assist researchers in engineering more efficient and stable alumina-based adsorbents for molybdenum adsorption used in medical radioisotope (
ions. These findings will assist researchers in engineering more efficient and stable alumina-based adsorbents for molybdenum adsorption used in medical radioisotope ( Mo/
Mo/ Tc) generators.
Tc) generators.
Benu, D. P.*; Earnshaw, J.*; Ashok, A.*; Tsuchiya, Kunihiko; Saptiama, I.*; Yuliarto, B.*; Suendo, V.*; Mukti, R. R.*; Fukumitsu, Nobuyoshi*; Ariga, Katsuhiko*; et al.
Bulletin of the Chemical Society of Japan, 94(2), p.502 - 507, 2021/02
Times Cited Count:11 Percentile:66.97(Chemistry, Multidisciplinary)no abstracts in English
Saptiama, I.*; Kaneti, Y. V.*; Yuliarto, B.*; Kumada, Hiroaki*; Tsuchiya, Kunihiko; Fujita, Yoshitaka; Malgras, V.*; Fukumitsu, Nobuyoshi*; Sakae, Takeji*; Hatano, Kentaro*; et al.
Chemistry; A European Journal, 25(18), p.4843 - 4855, 2019/03
Times Cited Count:15 Percentile:55.35(Chemistry, Multidisciplinary)The effective utilization of various biomolecules for creating a series of mesoporous boehmite ( -AlOOH) and gamma-alumina (
-AlOOH) and gamma-alumina ( -Al
-Al O
O ) nanosheets with unique hierarchical multilayered structures is demonstrated. The nature and concentration of the biomolecules strongly influence the degree of the crystallinity, the morphology, and the textural properties of the resulting
) nanosheets with unique hierarchical multilayered structures is demonstrated. The nature and concentration of the biomolecules strongly influence the degree of the crystallinity, the morphology, and the textural properties of the resulting  -AlOOH and
-AlOOH and  -Al
-Al O
O nanosheets, allowing for easy tuning. The hierarchical
nanosheets, allowing for easy tuning. The hierarchical  -AlOOH and
-AlOOH and  -Al
-Al O
O multilayered nanosheets synthesized by using biomolecules exhibit enhanced crystallinity, improved particle separation, and well-defined multilayered structures compared to those obtained without biomolecules. More impressively, these
multilayered nanosheets synthesized by using biomolecules exhibit enhanced crystallinity, improved particle separation, and well-defined multilayered structures compared to those obtained without biomolecules. More impressively, these  -AlOOH and
-AlOOH and  -Al
-Al O
O nanosheets possess high surface areas up to 425 and 371 m
nanosheets possess high surface areas up to 425 and 371 m /g, respectively, due to their mesoporous nature and hierarchical multilayered structure. When employed for molybdenum adsorption toward medical radioisotope production, the hierarchical
/g, respectively, due to their mesoporous nature and hierarchical multilayered structure. When employed for molybdenum adsorption toward medical radioisotope production, the hierarchical  -Al
-Al O
O multilayered nanosheets exhibit Mo adsorption capacities of 33.1
multilayered nanosheets exhibit Mo adsorption capacities of 33.1 40.8mg-Mo/g.
40.8mg-Mo/g.
Saptiama, I.*; Kaneti, Y. V.*; Suzuki, Yoshitaka; Tsuchiya, Kunihiko; Fukumitsu, Nobuyoshi*; Sakae, Takeji*; Kim, J.*; Kang, Y.-M.*; Ariga, Katsuhiko*; Yamauchi, Yusuke*
Small, 14(21), p.1800474_1 - 1800474_14, 2018/05
Times Cited Count:51 Percentile:89.35(Chemistry, Multidisciplinary)no abstracts in English
Saptiama, I.*; Kaneti, Y. V.*; Oveisi, H.*; Suzuki, Yoshitaka; Tsuchiya, Kunihiko; Takai, Kimiko*; Sakae, Takeji*; Pradhan, S.*; Hossain, M. S. A.*; Fukumitsu, Nobuyoshi*; et al.
Bulletin of the Chemical Society of Japan, 91(2), p.195 - 200, 2018/02
Times Cited Count:43 Percentile:81.72(Chemistry, Multidisciplinary)no abstracts in English
Saptiama, I.*; Kaneti, Y. V.*; Suzuki, Yumi*; Suzuki, Yoshitaka; Tsuchiya, Kunihiko; Sakae, Takeji*; Takai, Kimiko*; Fukumitsu, Nobuyoshi*; Alothman, Z. A.*; Hossain, M. S. A.*; et al.
Bulletin of the Chemical Society of Japan, 90(10), p.1174 - 1179, 2017/10
Times Cited Count:44 Percentile:79.84(Chemistry, Multidisciplinary)no abstracts in English
 Mo/
Mo/ Tc
TcFukumitsu, Nobuyoshi*; Yamauchi, Yusuke*; Kaneti, Y. V.*; Benu, D. P.*; Saptiama, I.*; Ariga, Katsuhiko*; Hatano, Kentaro*; Kumada, Hiroaki*; Fujita, Yoshitaka; Tsuchiya, Kunihiko
no journal, ,
no abstracts in English
 Mo/
Mo/ Tc
TcFukumitsu, Nobuyoshi*; Yamauchi, Yusuke*; Kaneti, Y. V.*; Benu, D. P.*; Saptiama, I.*; Ariga, Katsuhiko*; Hatano, Kentaro*; Kumada, Hiroaki*; Fujita, Yoshitaka; Tsuchiya, Kunihiko
no journal, ,
no abstracts in English
 Mo/
Mo/ Tc
TcFukumitsu, Nobuyoshi*; Yamauchi, Yusuke*; Kaneti, Y. V.*; Benu, D. P.*; Saptiama, I.*; Ariga, Katsuhiko*; Hatano, Kentaro*; Kumada, Hiroaki*; Fujita, Yoshitaka; Tsuchiya, Kunihiko
no journal, ,
no abstracts in English