Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kato, Tomoaki; Kozai, Naofumi; Tanaka, Kazuya; Kaplan, D. I.*; Utsunomiya, Satoshi*; Onuki, Toshihiko
Journal of Nuclear Science and Technology, 59(5), p.580 - 589, 2022/05
Times Cited Count:6 Percentile:55.57(Nuclear Science & Technology)This study reports the effect of fulvic acids, which is a natural organic substance generally contained in groundwater, on the oxidation states of radioactive iodine anions (iodide and iodate). Iodide and iodate are contained in the contaminated water of the Fukushima Daiichi Nuclear Power Plant and supposed to be removed by activated carbon (AC) via adsorption. When fulvic acids does not exist in the experimental system, the adsorption of iodide on AC was less than that of iodate and their oxidation states after the adsorption were not changed. When fulvic acids existed, a fraction of the adsorbed iodate was reduced to iodide. This result indicates that the reduction of the adsorbed iodate progresses during the storage of the spent AC.

 on Ni-Zn layered hydroxide salt and its application to removal of ReO
on Ni-Zn layered hydroxide salt and its application to removal of ReO
 as a surrogate of TcO
as a surrogate of TcO

Tanaka, Kazuya; Kozai, Naofumi; Yamasaki, Shinya*; Onuki, Toshihiko; Kaplan, D. I.*; Grambow, B.
Applied Clay Science, 182, p.105282_1 - 105282_8, 2019/12
Times Cited Count:24 Percentile:76.02(Chemistry, Physical)In this study, Ni-Zn layered hydroxide salt (LHS) was used for adsorption experiments of ReO
 , as a surrogate of TcO
, as a surrogate of TcO
 , in aqueous solutions with various initial Re and sodium salt concentrations. The maximum adsorption amount of Re was estimated at 127.7 mg/g (6.86
, in aqueous solutions with various initial Re and sodium salt concentrations. The maximum adsorption amount of Re was estimated at 127.7 mg/g (6.86  10
10 eq/g) by fitting adsorption isotherm of ReO
eq/g) by fitting adsorption isotherm of ReO
 to Langmuir plot. The adsorption of ReO
to Langmuir plot. The adsorption of ReO
 at neutral pH was a reversible process by anion exchange, and decreased with increasing Cl
at neutral pH was a reversible process by anion exchange, and decreased with increasing Cl , NO
, NO
 and SO
and SO
 in solution. EXAFS analysis indicated that ReO
in solution. EXAFS analysis indicated that ReO
 was adsorbed as an outer-sphere complex on Ni-Zn LHS. The Ni-Zn LHS is a more robust adsorbent for ReO
was adsorbed as an outer-sphere complex on Ni-Zn LHS. The Ni-Zn LHS is a more robust adsorbent for ReO
 than the Mg-Al LDH in terms of solution pH and tolerance to competing anions, and may be an effective alternative to the traditional and more limited method of removing aqueous TcO
than the Mg-Al LDH in terms of solution pH and tolerance to competing anions, and may be an effective alternative to the traditional and more limited method of removing aqueous TcO
 by reductive precipitation.
by reductive precipitation.
Lin, P.*; Xu, C.*; Kaplan, D. I.*; Chen, H.*; Yeager, C. M.*; Xing, W.*; Sun, L.*; Schwehr, K. A.*; Yamazaki, Hideo*; Kokubu, Yoko; et al.
Science of the Total Environment, 678, p.409 - 418, 2019/08
Times Cited Count:13 Percentile:40.84(Environmental Sciences)Nagasaki sediments containing bomb-derived Pu provided a unique opportunity to explore the long term geochemical behavior of Pu. Through a combination of selective extractions and molecular characterization via electrospray ionization Fourier-transform ion cyclotron resonance mass spectrometry, we determined that 55  3% of the
3% of the 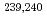 Pu was preferentially associated with more persistent organic matter compounds in Nagasaki sediments, particularly those natural organic matter (NOM) stabilized by Fe oxides. Other organic matter compounds served as a secondary sink of these
Pu was preferentially associated with more persistent organic matter compounds in Nagasaki sediments, particularly those natural organic matter (NOM) stabilized by Fe oxides. Other organic matter compounds served as a secondary sink of these 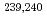 Pu (31
Pu (31  2% on average), and less than 20% of the
2% on average), and less than 20% of the 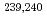 Pu was immobilized by inorganic mineral particles. While present long-term disposal and environmental remediation modeling assume that solubility limits and sorption to mineral surfaces control Pu subsurface mobility, our observations suggest that NOM undoubtedly plays an important role in sequestering Pu. Ignoring the role of NOM in controlling Pu fate and transport is not justified in most environmental systems.
Pu was immobilized by inorganic mineral particles. While present long-term disposal and environmental remediation modeling assume that solubility limits and sorption to mineral surfaces control Pu subsurface mobility, our observations suggest that NOM undoubtedly plays an important role in sequestering Pu. Ignoring the role of NOM in controlling Pu fate and transport is not justified in most environmental systems.
Tanaka, Kazuya; Kaplan, D. I.*; Onuki, Toshihiko
Applied Geochemistry, 85(Part B), p.119 - 120, 2017/10
Times Cited Count:0 Percentile:0.00(Geochemistry & Geophysics)We have prepared a special issue for Applied Geochemistry entitled "Transformation and Fate of Natural and Anthropogenic Radionuclides in the Environments". Here, we present 13 peer-reviewed articles on the general theme of natural and anthropogenic radionuclides in different environments. At the same time, these articles cover various topics of field research on the distribution of radionuclides, as well as laboratory experiments on adsorption and redox chemistry of these. The articles have been written by the attendees of the session at the Goldschmidt 2016 held in Yokohama, Japan, and by other authors who submitted their manuscripts to Applied Geochemistry focusing on the theme of the special issue.
Schwehr, K. A.*; Otosaka, Shigeyoshi; Merchel, S.*; Kaplan, D. I.*; Zhang, S.*; Xu, C.*; Li, H.-P.*; Ho, Y.-F.*; Yeager, C. M.*; Santschi, P. H.*; et al.
Science of the Total Environment, 497-498, p.671 - 678, 2014/11
Times Cited Count:16 Percentile:40.22(Environmental Sciences)A new, accurate and simple pH-dependent solvent extraction method combined with accelerator mass spectrometry (AMS) measurement for  I/
I/ I isotopes and iodine speciation (iodide, iodate, and organo-I) quantification in liquids of any ionic strength has been developed. We then validated the AMS method for activity concentration measurements with a recently developed gas chromatography mass spectrometry method for
I isotopes and iodine speciation (iodide, iodate, and organo-I) quantification in liquids of any ionic strength has been developed. We then validated the AMS method for activity concentration measurements with a recently developed gas chromatography mass spectrometry method for  I concentrations of 1 Bq/L or higher. This technique was applied to
I concentrations of 1 Bq/L or higher. This technique was applied to  I-contaminated groundwater from the Savannah River Site, USA, and demonstrated changes of
I-contaminated groundwater from the Savannah River Site, USA, and demonstrated changes of  I and
I and  I concentrations and speciation along a pH, redox potential, and organic carbon gradient. The data suggest that
I concentrations and speciation along a pH, redox potential, and organic carbon gradient. The data suggest that  I/
I/ I and species distribution is strongly pH dependent. The new method can now be applied to a wide range of chemically-diverse aquatic systems, including uncontaminated environments.
I and species distribution is strongly pH dependent. The new method can now be applied to a wide range of chemically-diverse aquatic systems, including uncontaminated environments.
 O
O -dependent iodide oxidation
-dependent iodide oxidationLi, H.-P.*; Yeager, C. M.*; Brinkmeyer, R.*; Zhang, S.*; Ho, Y.-F.*; Xu, C.*; Jones, W. L.*; Schwehr, K. A.*; Otosaka, Shigeyoshi; Roberts, K. A.*; et al.
Environmental Science & Technology, 46(9), p.4837 - 4844, 2012/03
Times Cited Count:58 Percentile:78.99(Engineering, Environmental)In order to develop an understanding of the role that microorganisms play in the transport of  I in soil-water systems, naturally occurring bacteria isolated from the F-area subsurface of the Savannah River Site (SRS) were assessed for iodide oxidizing activity. Spent liquid medium from a number of SRS bacterial cultures enhanced iodide oxidation 2-10 fold in the presence of hydrogen peroxide (H
I in soil-water systems, naturally occurring bacteria isolated from the F-area subsurface of the Savannah River Site (SRS) were assessed for iodide oxidizing activity. Spent liquid medium from a number of SRS bacterial cultures enhanced iodide oxidation 2-10 fold in the presence of hydrogen peroxide (H O
O ). From a time-series measurements of peroxidase activities and organic acid concentrations, it was hypothesized that microbial organic acid exudate promoted iodide oxidation via following mechanisms; (1) organic acids interact with H
). From a time-series measurements of peroxidase activities and organic acid concentrations, it was hypothesized that microbial organic acid exudate promoted iodide oxidation via following mechanisms; (1) organic acids interact with H O
O to form strong iodide oxidizing agents, peroxy carboxylic acids, and (2) organic acid secretion led to enhanced rates of H
to form strong iodide oxidizing agents, peroxy carboxylic acids, and (2) organic acid secretion led to enhanced rates of H O
O -dependent iodide oxidation by lowering the pH of the culture medium.
-dependent iodide oxidation by lowering the pH of the culture medium.
 I) by soil organic matter and possible consequences of the remedial action at Savannah River Site
I) by soil organic matter and possible consequences of the remedial action at Savannah River SiteXu, C.*; Miller, E. J.*; Zhang, S.*; Li, H.-P.*; Ho, Y.-F.*; Schwehr, K. A.*; Kaplan, D. I.*; Otosaka, Shigeyoshi; Roberts, K. A.*; Brinkmeyer, R.*; et al.
Environmental Science & Technology, 45(23), p.9975 - 9983, 2011/12
Times Cited Count:77 Percentile:84.93(Engineering, Environmental)In order to investigate accumulation process of iodine-129 ( I) in a contaminated F-Area groundwater plume of the US Savannah River Site, soil resuspension experiments simulating surface runoff and erosion events were conducted. Results showed that 72
I) in a contaminated F-Area groundwater plume of the US Savannah River Site, soil resuspension experiments simulating surface runoff and erosion events were conducted. Results showed that 72 77% of the newly-introduced iodide was irreversibly sequestered into the organic-rich soil, while the rest was transformed into colloidal and dissolved organo-iodine by the soil. Laboratory iodination of the soil indicated a preferential incorporation of inorganic iodine into soil organic matter (SOM) at acidic pH (3
77% of the newly-introduced iodide was irreversibly sequestered into the organic-rich soil, while the rest was transformed into colloidal and dissolved organo-iodine by the soil. Laboratory iodination of the soil indicated a preferential incorporation of inorganic iodine into soil organic matter (SOM) at acidic pH (3 4), except for the iodination catalyzed by lactoperoxidase, which favors more alkaline conditions. From this result, we concluded that under very acidic conditions, abiotic iodination of SOM was predominant, while under less acidic conditions (pH
4), except for the iodination catalyzed by lactoperoxidase, which favors more alkaline conditions. From this result, we concluded that under very acidic conditions, abiotic iodination of SOM was predominant, while under less acidic conditions (pH  5), microbial enzymatically-assisted iodination took over.
5), microbial enzymatically-assisted iodination took over.
 I) at the Savannah River Site?
I) at the Savannah River Site?Xu, C.*; Zhang, S.*; Ho, Y.-F.*; Miller, E. J.*; Roberts, K. A.*; Li, H.-P.*; Schwehr, K. A.*; Otosaka, Shigeyoshi; Kaplan, D. I.*; Brinkmeyer, R.*; et al.
Geochimica et Cosmochimica Acta, 75(19), p.5716 - 5735, 2011/10
Times Cited Count:65 Percentile:82.71(Geochemistry & Geophysics)In order to understand the effect of soil organic matter (SOM) on the mobility of iodine in the vicinity of the F-area seepage basin at the U.S. Department Energy's Savannah River Site (SRS), relationships between radioiodine/iodine concentration and properties of SOM (e.g., degree of humification, aromaticity, and molecular weight) were examined. Analyses were carried out for four sequential extracts of SOM (freshwater, alkaline, glycerol, and citric-alkaline solutions). Iodine in SOM was selectively bound to a small-size aromatic subunit (less than 10 kDa), and majority of water soluble  I was associated with a low molecular weight amphiphilic organic carrier (13.5-15 kDa). From these results, it was suggested that (1) SOM behaved as a sink as well as a source for iodine at the SRS, and (2) the function of SOM varies with groundwater chemistry.
I was associated with a low molecular weight amphiphilic organic carrier (13.5-15 kDa). From these results, it was suggested that (1) SOM behaved as a sink as well as a source for iodine at the SRS, and (2) the function of SOM varies with groundwater chemistry.
 I and
I and  I species in an acidic groundwater plume at the Savannah River Site
I species in an acidic groundwater plume at the Savannah River SiteOtosaka, Shigeyoshi; Schwehr, K. A.*; Kaplan, D. I.*; Roberts, K. A.*; Zhang, S.*; Xu, C.*; Li, H.-P.*; Ho, Y.-F.*; Brinkmeyer, R.*; Yeager, C. M.*; et al.
Science of the Total Environment, 409(19), p.3857 - 3865, 2011/09
Times Cited Count:70 Percentile:82.86(Environmental Sciences)Spatial distributions of concentrations and speciation of radioiodine ( I) and stable iodine (
I) and stable iodine ( I) in groundwater in the vicinity of the F-area seepage basin at the U.S. Department Energy of Savannah River Site were investigated.
I) in groundwater in the vicinity of the F-area seepage basin at the U.S. Department Energy of Savannah River Site were investigated.  I concentration in groundwater was 8.6 Bq/L immediately downstream of the seepage basin (well FSB-95DR), and decreased with distance from the infiltration basin.
I concentration in groundwater was 8.6 Bq/L immediately downstream of the seepage basin (well FSB-95DR), and decreased with distance from the infiltration basin.  I concentration decreased similarly to that of
I concentration decreased similarly to that of  I. Although there was no potential
I. Although there was no potential  I source in wastes in the basin,
I source in wastes in the basin,  I also showed a similar gradient to that of
I also showed a similar gradient to that of  I. High concentrations of
I. High concentrations of  I or
I or  I were not detected in groundwater collected from wells located outside of the mixed waste plume of this area. The high iodide concentrations in groundwater near the basin were presumed to be caused by dissolution of iodide from soil due to gradually increasing of pH values in the last decade.
I were not detected in groundwater collected from wells located outside of the mixed waste plume of this area. The high iodide concentrations in groundwater near the basin were presumed to be caused by dissolution of iodide from soil due to gradually increasing of pH values in the last decade.