Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Furuya, Shunsuke*; Matsuo, Mamoru; Kato, Takeo*
Physical Review B, 110(16), p.165129_1 - 165129_13, 2024/10
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)Hosokawa, Kaiji*; Yama, Masaki*; Matsuo, Mamoru; Kato, Takeo*
Physical Review B, 110(3), p.035309_1 - 035309_12, 2024/07
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)Funato, Takumi*; Matsuo, Mamoru; Kato, Takeo*
Physical Review Letters, 132(23), p.236201_1 - 236201_7, 2024/06
Times Cited Count:4 Percentile:85.16(Physics, Multidisciplinary)Hirooka, Shun; Horii, Yuta; Sunaoshi, Takeo*; Uno, Hiroki*; Yamada, Tadahisa*; Vauchy, R.; Hayashizaki, Kohei; Nakamichi, Shinya; Murakami, Tatsutoshi; Kato, Masato
Journal of Nuclear Science and Technology, 60(11), p.1313 - 1323, 2023/11
Times Cited Count:5 Percentile:80.64(Nuclear Science & Technology)Additive MOX pellets are fabricated by a conventional dry powder metallurgy method. Nd O
O and Sm
and Sm O
O are chosen as the additive materials to simulate the corresponding soluble fission products dispersed in MOX. Shrinkage curves of the MOX pellets are obtained by dilatometry, which reveal that the sintering temperature is shifted toward a value higher than that of the respective regular MOX. The additives, however, promote grain growth and densification, which can be explained by the effect of oxidized uranium cations covering to a pentavalent state. Ceramography reveals large agglomerates after sintering, and Electron Probe Micro-Analysis confirms that inhomogeneous elemental distribution, whereas XRD reveals a single face-centered cubic phase. Finally, by grinding and re-sintering the specimens, the cation distribution homogeneity is significantly improved, which can simulate spent nuclear fuels with soluble fission products.
are chosen as the additive materials to simulate the corresponding soluble fission products dispersed in MOX. Shrinkage curves of the MOX pellets are obtained by dilatometry, which reveal that the sintering temperature is shifted toward a value higher than that of the respective regular MOX. The additives, however, promote grain growth and densification, which can be explained by the effect of oxidized uranium cations covering to a pentavalent state. Ceramography reveals large agglomerates after sintering, and Electron Probe Micro-Analysis confirms that inhomogeneous elemental distribution, whereas XRD reveals a single face-centered cubic phase. Finally, by grinding and re-sintering the specimens, the cation distribution homogeneity is significantly improved, which can simulate spent nuclear fuels with soluble fission products.
Yama, Masaki*; Matsuo, Mamoru; Kato, Takeo*
Physical Review B, 108(14), p.144430_1 - 144430_15, 2023/10
Times Cited Count:4 Percentile:50.15(Materials Science, Multidisciplinary)Fukuzawa, K.*; Kato, Takeo*; Matsuo, Mamoru; Jonckheere, T.*; Rech, J.*; Martin, T.*
Physical Review B, 108(13), p.134429_1 - 134429_9, 2023/10
Times Cited Count:1 Percentile:13.37(Materials Science, Multidisciplinary)Sato, Tetsuya*; Oue, Daigo*; Matsuo, Mamoru; Kato, Takeo*
Physical Review B, 108(9), p.094428_1 - 094428_10, 2023/09
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)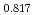 Pu
Pu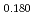 Am
Am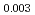 O
O uranium-plutonium-americium mixed oxides at 1573, 1773, and 1873 K
uranium-plutonium-americium mixed oxides at 1573, 1773, and 1873 KVauchy, R.; Sunaoshi, Takeo*; Hirooka, Shun; Nakamichi, Shinya; Murakami, Tatsutoshi; Kato, Masato
Journal of Nuclear Materials, 580, p.154416_1 - 154416_11, 2023/07
Times Cited Count:9 Percentile:94.10(Materials Science, Multidisciplinary)Yama, Masaki*; Matsuo, Mamoru; Kato, Takeo*
Physical Review B, 107(17), p.174414_1 - 174414_15, 2023/05
Times Cited Count:5 Percentile:50.15(Materials Science, Multidisciplinary)Sato, Tetsuya*; Kato, Takeo*; Oue, Daigo*; Matsuo, Mamoru
Physical Review B, 107(18), p.L180406_1 - L180406_6, 2023/05
Times Cited Count:2 Percentile:27.36(Materials Science, Multidisciplinary)Tajima, Hiroyuki*; Oue, Daigo*; Matsuo, Mamoru; Kato, Takeo*
PNAS NEXUS (Internet), 2(3), p.pgad045_1 - pgad045_6, 2023/03
Ishikawa, Takuto*; Matsuo, Mamoru; Kato, Takeo*
Physical Review B, 107(5), p.054426_1 - 054426_9, 2023/02
Times Cited Count:1 Percentile:13.37(Materials Science, Multidisciplinary)Funato, Takumi*; Kato, Takeo*; Matsuo, Mamoru
Physical Review B, 106(14), p.144418_1 - 144418_10, 2022/10
Times Cited Count:4 Percentile:34.41(Materials Science, Multidisciplinary)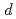 -wave superconductor/ferromagnetic insulator bilayer systems
-wave superconductor/ferromagnetic insulator bilayer systemsOminato, Yuya*; Yamakage, Ai*; Kato, Takeo*; Matsuo, Mamoru
Physical Review B, 105(20), p.205406_1 - 205406_7, 2022/05
Times Cited Count:12 Percentile:73.42(Materials Science, Multidisciplinary)Sato, Tetsuya*; Tatsuno, Masahiro*; Matsuo, Mamoru; Kato, Takeo*
Journal of Magnetism and Magnetic Materials, 546, p.168814_1 - 168814_6, 2022/03
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)Izumida, Wataru*; Okuyama, Rin*; Sato, Kentaro*; Kato, Takeo*; Matsuo, Mamoru
Physical Review Letters, 128(1), p.017701_1 - 017701_6, 2022/01
Times Cited Count:7 Percentile:59.84(Physics, Multidisciplinary)Yamamoto, Tsuyoshi*; Kato, Takeo*; Matsuo, Mamoru
Physical Review B, 104(12), p.L121401_1 - L121401_5, 2021/09
Times Cited Count:7 Percentile:42.22(Materials Science, Multidisciplinary)Yama, Masaki*; Tatsuno, Masahiro*; Kato, Takeo*; Matsuo, Mamoru
Physical Review B, 104(5), p.054410_1 - 054410_9, 2021/08
Times Cited Count:9 Percentile:47.11(Materials Science, Multidisciplinary) and (U,Pu,Am,Np)O
and (U,Pu,Am,Np)O
Hirooka, Shun; Matsumoto, Taku; Kato, Masato; Sunaoshi, Takeo*; Uno, Hiroki*; Yamada, Tadahisa*
Journal of Nuclear Materials, 542, p.152424_1 - 152424_9, 2020/12
Times Cited Count:12 Percentile:75.34(Materials Science, Multidisciplinary)The measurement of oxygen potential was conducted at 1,673, 1,773, and 1,873 K for (U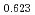 Pu
Pu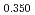 Am
Am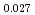 )O
)O and at 1,873 and 1,923 K for (U
and at 1,873 and 1,923 K for (U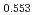 Pu
Pu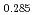 Am
Am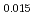 Np
Np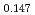 )O
)O by using a thermo-gravimeter and an oxygen sensor. Am inclusion in terms of substituting the U significantly increased the oxygen potential. Similarly, the inclusion of Np as a substitute for U increased the oxygen potential; however, the effect was not as large as that with the Pu or Am addition at the same rate. The results were analyzed via defect chemistry and certain defect formations were suggested in the reducing region and the near-stoichiometric region by plotting the relationship between PO
by using a thermo-gravimeter and an oxygen sensor. Am inclusion in terms of substituting the U significantly increased the oxygen potential. Similarly, the inclusion of Np as a substitute for U increased the oxygen potential; however, the effect was not as large as that with the Pu or Am addition at the same rate. The results were analyzed via defect chemistry and certain defect formations were suggested in the reducing region and the near-stoichiometric region by plotting the relationship between PO and the deviation from the stoichiometry. The equilibrium constants of the defect reactions were arranged to reproduce the experiment such that Am/Np contents were included in the entropy with coefficients fitting the experimental data.
and the deviation from the stoichiometry. The equilibrium constants of the defect reactions were arranged to reproduce the experiment such that Am/Np contents were included in the entropy with coefficients fitting the experimental data.
 at high temperatures of 1673-1873 K
at high temperatures of 1673-1873 KWatanabe, Masashi; Kato, Masato; Sunaoshi, Takeo*
Journal of Nuclear Materials, 542, p.152472_1 - 152472_7, 2020/12
Times Cited Count:3 Percentile:27.12(Materials Science, Multidisciplinary)The oxygen self-diffusion coefficients in near stoichiometric (U,Pu)O at high temperatures were successfully measured by thermogravimetry combined with the oxygen isotope exchange method. The activation energy for oxygen diffusion in the stoichiometric composition of (U,Pu)O
at high temperatures were successfully measured by thermogravimetry combined with the oxygen isotope exchange method. The activation energy for oxygen diffusion in the stoichiometric composition of (U,Pu)O was evaluated from experimental data, and the value was determined to be 248 kJ/mol. In addition, the defect migration energies of (U,Pu)O
was evaluated from experimental data, and the value was determined to be 248 kJ/mol. In addition, the defect migration energies of (U,Pu)O were derived, and the oxygen self-diffusion coefficients were evaluated using these. As a result, good agreement was found between the experimental data and the oxygen self-diffusion coefficients calculated using the defect migration energies.
were derived, and the oxygen self-diffusion coefficients were evaluated using these. As a result, good agreement was found between the experimental data and the oxygen self-diffusion coefficients calculated using the defect migration energies.