Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Takasaki, Koji; Yasumune, Takashi; Yamaguchi, Yukako; Hashimoto, Makoto; Maeda, Koji; Kato, Masato
Journal of Nuclear Science and Technology, 60(11), p.1437 - 1446, 2023/11
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)The aerodynamic radioactive median diameter (AMAD) is necessary information to assess the internal exposure. On June 6, 2017, at a plutonium handling facility in Oarai site of Japan Atomic Energy Agency (JAEA), during the inspection work of a storage container that contains nuclear fuel materials, accidental contamination occurred and five workers inhaled radioactive materials including plutonium. Some smear papers and an air sampling filter were measured with the imaging plate, and we conservatively estimated minimum AMADs for two cases, plutonium nitrate and plutonium dioxide. As a result of AMAD estimation, even excluding a giant particle of a smear sample, the minimum AMADs of plutonium nitrate from smear papers were 4.3 - 11.3  m and those of plutonium dioxide were 5.6 - 14.1
m and those of plutonium dioxide were 5.6 - 14.1  m. Also, the minimum AMAD of plutonium nitrate from an air sampling filter was 3.0
m. Also, the minimum AMAD of plutonium nitrate from an air sampling filter was 3.0  m and that of plutonium dioxide was 3.9
m and that of plutonium dioxide was 3.9  m.
m.
Takeda, Yukiharu; Saito, Yuji; Saito, Hiroyuki; Machida, Akihiko; Aoki, Katsutoshi; Yamagami, Hiroshi; Muro, Takayuki*; Kato, Yukako*; Kinoshita, Toyohiko*
Physical Review B, 84(15), p.153102_1 - 153102_4, 2011/10
Times Cited Count:7 Percentile:32.89(Materials Science, Multidisciplinary)We have performed soft X-ray emission spectroscopy (SXES) and soft X-ray absorption spectroscopy (SXAS) experiments on aluminum hydride 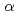 -AlH
-AlH . The occupied and unoccupied electronic states of the Al 3
. The occupied and unoccupied electronic states of the Al 3 partial density of states are obtained experimentally. By comparing the data from Al metal and
partial density of states are obtained experimentally. By comparing the data from Al metal and 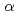 -AlH
-AlH , a band gap with a few eV is found for
, a band gap with a few eV is found for 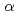 -AlH
-AlH . In addition, the occupied states of
. In addition, the occupied states of 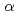 -AlH
-AlH have a larger spectral intensity than that of Al metal, indicating an increase in the number of electrons with the Al 3
have a larger spectral intensity than that of Al metal, indicating an increase in the number of electrons with the Al 3 character through Al-H bond formations. The results of a band-structure calculation account for the formation of the energy gap and the increase of the Al 3
character through Al-H bond formations. The results of a band-structure calculation account for the formation of the energy gap and the increase of the Al 3 electrons qualitatively. This suggests that a covalent-like nature is important to the Al-H bond in
electrons qualitatively. This suggests that a covalent-like nature is important to the Al-H bond in 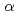 -AlH
-AlH .
.
Kamakura, Nozomu; Takeda, Yukiharu; Saito, Yuji; Yamagami, Hiroshi; Tsubota, Masami*; Paik, B.*; Ichikawa, Takayuki*; Kojima, Yoshitsugu*; Muro, Takayuki*; Kato, Yukako*; et al.
Physical Review B, 83(3), p.033103_1 - 033103_4, 2011/01
Times Cited Count:5 Percentile:25.35(Materials Science, Multidisciplinary)The electronic structure of lithium amide, which is lightweight complex hydride expected as a high-capacity hydrogen storage material, is investigated by N 1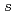 soft X-ray emission spectroscopy (XES) and absorption spectroscopy (XAS). The overall feature of the electronic structure of lithium amide by the XES and XAS is consistent with the band calculation, while the strongly hybridized state with H 1
soft X-ray emission spectroscopy (XES) and absorption spectroscopy (XAS). The overall feature of the electronic structure of lithium amide by the XES and XAS is consistent with the band calculation, while the strongly hybridized state with H 1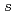 is located at higher binding energy than the band calculation.
is located at higher binding energy than the band calculation.
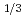 NbS
NbS
Guo, F. Z.*; Matsushita, Tomohiro*; Kobayashi, Keisuke*; Matsui, Fumihiko*; Kato, Yukako*; Daimon, Hiroshi*; Koyano, Mikio*; Yamamura, Yasuhisa*; Tsuji, Toshihide*; Saito, Yuji
Journal of Applied Physics, 99(2), p.024907_1 - 024907_3, 2006/01
Times Cited Count:8 Percentile:31.28(Physics, Applied)Stereoatomscope was used to study the atomic arrangements of intercalation compound Fe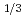 NbS
NbS . The three-dimensional atomic arrangements around different kinds of atoms (Nb and Fe) are visualized by taking the photoelectron angular distribution (PEAD) patterns at clockwise and counterclockwise circularly polarized lights. Atomic distances between the emitters and the scatterers are obtained from the PEAD patterns by measuring the rotation angles of the forward focusing peaks. The applications of stereoatomscope to intercalation compound show the possibility to build an ultimate microscope for scientist.
. The three-dimensional atomic arrangements around different kinds of atoms (Nb and Fe) are visualized by taking the photoelectron angular distribution (PEAD) patterns at clockwise and counterclockwise circularly polarized lights. Atomic distances between the emitters and the scatterers are obtained from the PEAD patterns by measuring the rotation angles of the forward focusing peaks. The applications of stereoatomscope to intercalation compound show the possibility to build an ultimate microscope for scientist.
Matsushita, Tomohiro*; Guo, F. Z.*; Agui, Akane; Kato, Yukako*; Matsui, Fumihiko*; Daimon, Hiroshi*
no journal, ,
no abstracts in English
 by soft X-ray emission spectroscopy
by soft X-ray emission spectroscopyTakeda, Yukiharu; Okane, Tetsuo; Fujimori, Shinichi; Yasui, Akira; Saito, Yuji; Yamagami, Hiroshi; Saito, Hiroyuki; Muro, Takayuki*; Kato, Yukako*; Kinoshita, Toyohiko*
no journal, ,
no abstracts in English
 using soft X-ray emission and absorption spectroscopy
using soft X-ray emission and absorption spectroscopyTakeda, Yukiharu; Saito, Yuji; Yamagami, Hiroshi; Saito, Hiroyuki; Machida, Akihiko; Aoki, Katsutoshi; Muro, Takayuki*; Kato, Yukako*; Kinoshita, Toyohiko*
no journal, ,
no abstracts in English