Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hata, Kuniki; Lin, M.*; Yokoya, Akinari*; Fujii, Kentaro*; Yamashita, Shinichi*; Muroya, Yusa*; Katsumura, Yosuke*
Hoshasen Kagaku (Internet), (103), p.29 - 34, 2017/04
Reactivity of edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), which is known to show high antioxidative properties, with oxidative species, such as hydroxyl radical (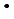 OH) and azide radical (N
OH) and azide radical (N
 ), was investigated by a pulse radiolysis experiment, and generation behavior of edaravone radicals produced through these reactions were observed. It was shown that OH-adducts are produced by the reaction with
), was investigated by a pulse radiolysis experiment, and generation behavior of edaravone radicals produced through these reactions were observed. It was shown that OH-adducts are produced by the reaction with 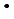 OH in contrast to the other oxidative radicals, which react with edaravone by an electron transfer reaction. Chemical repair properties of edaravone against DNA lesions produced by reactions of DNA with oxidative species were also investigated by a pulse radiolysis experiment with deoxyguanosine monophosphate (dGMP) and a
OH in contrast to the other oxidative radicals, which react with edaravone by an electron transfer reaction. Chemical repair properties of edaravone against DNA lesions produced by reactions of DNA with oxidative species were also investigated by a pulse radiolysis experiment with deoxyguanosine monophosphate (dGMP) and a  -radiolysis experiment with plasmid DNA solutions. It was observed that edaravone reduced dGMP radicals just after produced in a dilute aqueous solution and inhibited some base lesions on plasmid DNA more effectively than single strand breaks. These results show that edaravone may protect living system from oxidative stress, such as radiation, by not only scavenging oxidative species but also reducing precursors of DNA leisons.
-radiolysis experiment with plasmid DNA solutions. It was observed that edaravone reduced dGMP radicals just after produced in a dilute aqueous solution and inhibited some base lesions on plasmid DNA more effectively than single strand breaks. These results show that edaravone may protect living system from oxidative stress, such as radiation, by not only scavenging oxidative species but also reducing precursors of DNA leisons.
Yang, S.*; Katsumura, Yosuke*; Yamashita, Shinichi*; Matsuura, Chihiro*; Hiroishi, Daisuke*; Lertnaisat, P.*; Taguchi, Mitsumasa
Radiation Physics and Chemistry, 123, p.14 - 19, 2016/06
Times Cited Count:2 Percentile:17.78(Chemistry, Physical) -radiolysis of boiling water has been investigated. The G-value of H
-radiolysis of boiling water has been investigated. The G-value of H evolution was found to be very sensitive to the purity of water. In high-purity water, both H
evolution was found to be very sensitive to the purity of water. In high-purity water, both H and O
and O gases were formed in the stoichiometric ratio of 2:1; a negligible amount of H
gases were formed in the stoichiometric ratio of 2:1; a negligible amount of H O
O remained in the liquid phase. The G-values of H
remained in the liquid phase. The G-values of H and O
and O gas evolution depend on the dose rate: lower dose rates produce larger yields. To clarify the importance of the interface between liquid and gas phase for gas evolution, the gas evolution under Ar gas bubbling was measured. A large amount of H
gas evolution depend on the dose rate: lower dose rates produce larger yields. To clarify the importance of the interface between liquid and gas phase for gas evolution, the gas evolution under Ar gas bubbling was measured. A large amount of H was detected, similar to the radiolysis of boiling water. The evolution of gas was enhanced in a 0.5 M NaCl aqueous solution. Deterministic chemical kinetics simulations elucidated the mechanism of radiolysis in boiling water.
was detected, similar to the radiolysis of boiling water. The evolution of gas was enhanced in a 0.5 M NaCl aqueous solution. Deterministic chemical kinetics simulations elucidated the mechanism of radiolysis in boiling water.
Iwamatsu, Kazuhiro*; Muroya, Yusa*; Yamashita, Shinichi*; Kimura, Atsushi; Taguchi, Mitsumasa; Katsumura, Yosuke*
Radiation Physics and Chemistry, 119, p.213 - 217, 2016/02
Times Cited Count:1 Percentile:9.56(Chemistry, Physical)A quick measurement system of a continuous absorption spectrum covering a wide range from 200 to 950 nm was constructed by employing an optical multi-channel detector. Ion beam pulse radiolysis with 12.5 MeV/u He, 18.3 MeV/u C and 17.5 MeV/u Ne ions were performed with the measurement system. Transient absorption spectrum of (SCN)
 was clearly observed in KSCN aqueous solutions within a few minutes in spite of their very small absorbance, demonstrating high sensitivity of 0.001-0.003 in absorbance in the range from 260 to 660 nm as well as short measurement time of a few minutes. Two different absorption peaks attributed to Br
was clearly observed in KSCN aqueous solutions within a few minutes in spite of their very small absorbance, demonstrating high sensitivity of 0.001-0.003 in absorbance in the range from 260 to 660 nm as well as short measurement time of a few minutes. Two different absorption peaks attributed to Br
 and Br
and Br
 were observed simultaneously in NaBr aqueous solutions, showing powerfulness of the measurement system in overviewing chemical kinetics under ion beam irradiation especially in not well investigated chemical systems.
were observed simultaneously in NaBr aqueous solutions, showing powerfulness of the measurement system in overviewing chemical kinetics under ion beam irradiation especially in not well investigated chemical systems.
Yamashita, Shinichi*; Iwamatsu, Kazuhiro; Maehashi, Yuki*; Taguchi, Mitsumasa; Hata, Kuniki; Muroya, Yusa*; Katsumura, Yosuke*
RSC Advances (Internet), 5(33), p.25877 - 25886, 2015/02
Times Cited Count:13 Percentile:35.89(Chemistry, Multidisciplinary)Pulse radiolysis experiments were carried out to observe transient absorptions of reaction intermediates produced in N O
O and Ar-saturated aqueous solutions containing 0.9-900 mM NaBr. The most important species among the reaction intermediates are BrOH
and Ar-saturated aqueous solutions containing 0.9-900 mM NaBr. The most important species among the reaction intermediates are BrOH 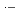 and Br
and Br
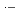 , which commonly have absorption peaks around 360 nm. The experimental results were compared with the results of simulation based on a spur diffusion model. Each of several complicated sequential radiation-induced chemical reactions was carefully considered, optimizing its rate constant within a range of reported values. All the experimental results were able to be universally reproduced by the simulation, assuming a reaction not yet reported, 2BrOH
, which commonly have absorption peaks around 360 nm. The experimental results were compared with the results of simulation based on a spur diffusion model. Each of several complicated sequential radiation-induced chemical reactions was carefully considered, optimizing its rate constant within a range of reported values. All the experimental results were able to be universally reproduced by the simulation, assuming a reaction not yet reported, 2BrOH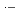
 Br
Br + 2OH
+ 2OH , with a rate constant of 3.8
, with a rate constant of 3.8  10
10 M
M s
s , which is significant only within 10 micro-s for rather high bromide concentrations (
, which is significant only within 10 micro-s for rather high bromide concentrations ( 10 mM). Primary
10 mM). Primary 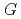 values, which are yields after sufficient diffusion from the spur to the perimeter region during 100 ns, of major water decomposition products, as well as of the reaction intermediates, were calculated for N
values, which are yields after sufficient diffusion from the spur to the perimeter region during 100 ns, of major water decomposition products, as well as of the reaction intermediates, were calculated for N O
O and Ar-saturated conditions as a function of NaBr concentration.
and Ar-saturated conditions as a function of NaBr concentration.
Hata, Kuniki; Urushibara, Ayumi*; Yamashita, Shinichi*; Lin, M.*; Muroya, Yusa*; Shikazono, Naoya; Yokoya, Akinari; Fu, H.*; Katsumura, Yosuke*
Journal of Radiation Research, 56(1), p.59 - 66, 2015/01
Times Cited Count:6 Percentile:27.68(Biology)Tran Duy, T.*; Sawada, Shinichi; Hasegawa, Shin; Yoshimura, Kimio; Oba, Yojiro*; Onuma, Masato*; Katsumura, Yosuke*; Maekawa, Yasunari
Macromolecules, 47(7), p.2373 - 2383, 2014/04
Times Cited Count:32 Percentile:71.78(Polymer Science)The hierarchical structures of graft-type ETFE-based polymer electrolyte membranes (ETFE-PEMs) were investigated using small- and ultrasmall-angle X-ray cattering experiments. The ETFE-PEMs with IECs  2.4 mmol/g possessed conducting graft domains around lamellar crystals, with a d-spacing of 21.8-29.1 nm, and oriented crystallites with short and long correlation distances of 218-320 and 903-1124 nm, respectively. The membranes with IECs
2.4 mmol/g possessed conducting graft domains around lamellar crystals, with a d-spacing of 21.8-29.1 nm, and oriented crystallites with short and long correlation distances of 218-320 and 903-1124 nm, respectively. The membranes with IECs  2.7 mmol/g showed a new phase of crystallite network domains with a d-range of 225-256 nm, indicating a phase transition from oriented crystallite to crystallite network structures in the IEC range of 2.4-2.7 mmol/g. Noted that for the ETFE-PEMs with high IECs higher conductivity at 30% RH and compatible tensile strengths at 100% RH and 80
2.7 mmol/g showed a new phase of crystallite network domains with a d-range of 225-256 nm, indicating a phase transition from oriented crystallite to crystallite network structures in the IEC range of 2.4-2.7 mmol/g. Noted that for the ETFE-PEMs with high IECs higher conductivity at 30% RH and compatible tensile strengths at 100% RH and 80  C, compared with Nafion, originated from the well-interconnected ion channels around the crystallites and the remaining lamellar crystals and crystallites, respectively.
C, compared with Nafion, originated from the well-interconnected ion channels around the crystallites and the remaining lamellar crystals and crystallites, respectively.
Tran, D. T.; Sawada, Shinichi; Hasegawa, Shin; Katsumura, Yosuke*; Maekawa, Yasunari
Journal of Membrane Science, 447, p.19 - 25, 2013/11
Times Cited Count:38 Percentile:75.39(Engineering, Chemical)Relative humidity (RH) dependence of the proton conduction and mechanical properties of poly(ethylene-co-tetrafluoroethylene) (ETFE)-based radiation grafted polymer electrolyte membranes (PEMs) were investigated in a wide ion exchange capacity (IEC) range at 80  C. The proton conductivities of the ETFE-based PEMs for IECs of 1.3-2.9 mmol/g were 0.001-0.013 S/cm at 30% RH. These PEMs have conductivities that are less dependent on RH than aromatic-hydrocarbon-polymer based PEMs. The ETFE PEM (IEC
C. The proton conductivities of the ETFE-based PEMs for IECs of 1.3-2.9 mmol/g were 0.001-0.013 S/cm at 30% RH. These PEMs have conductivities that are less dependent on RH than aromatic-hydrocarbon-polymer based PEMs. The ETFE PEM (IEC  2.4 mmol/g) showed higher tensile strength than Nafion at 100% RH. It was revealed that the mechanical strength and proton conductivity were clearly related to PEM crystallinities.
2.4 mmol/g) showed higher tensile strength than Nafion at 100% RH. It was revealed that the mechanical strength and proton conductivity were clearly related to PEM crystallinities.
Nuryanthi, N.*; Yamaki, Tetsuya; Koshikawa, Hiroshi; Asano, Masaharu; Sawada, Shinichi; Hasegawa, Shin; Maekawa, Yasunari; Katsumura, Yosuke*
Nuclear Instruments and Methods in Physics Research B, 314, p.95 - 98, 2013/11
Times Cited Count:3 Percentile:25.64(Instruments & Instrumentation)Our efforts have been focused on ion-track etched membranes of poly(vinylidene fluoride) (PVDF). This study deals with the effect of the transmembrane potential applied during the conductometry in order to offer a higher degree of freedom to control the pore size. We can say that higher voltage application during the conductometry would accelerate the etching in the tracks. The electrophoretic migration of dissolved products occurring out of each pore might be one of the reasons for this enhanced pore evolution and growth.
Hata, Kuniki; Urushibara, Ayumi; Yamashita, Shinichi; Shikazono, Naoya; Yokoya, Akinari; Katsumura, Yosuke*
Biochemical and Biophysical Research Communications, 434(2), p.341 - 345, 2013/05
Times Cited Count:8 Percentile:22.50(Biochemistry & Molecular Biology)Nuryanthi, N.*; Yamaki, Tetsuya; Koshikawa, Hiroshi; Asano, Masaharu; Sawada, Shinichi; Hasegawa, Shin; Maekawa, Yasunari; Katsumura, Yosuke*
Transactions of the Materials Research Society of Japan, 38(1), p.105 - 108, 2013/03
We report here how conditions of the conductometric analysis affected the etching characteristics of 25  m-thick poly(vinylidene fluoride) film irradiated with 450 MeV
m-thick poly(vinylidene fluoride) film irradiated with 450 MeV  Xe ions. The etching was performed in a 9 mol dm
Xe ions. The etching was performed in a 9 mol dm aqueous potassium hydroxide solution at 80
aqueous potassium hydroxide solution at 80 C in a conductometric cell. According to the scanning electron microscope observations, the ion-track membrane obtained with an applied AC voltage of 1.0 V had the surface pores of 168
C in a conductometric cell. According to the scanning electron microscope observations, the ion-track membrane obtained with an applied AC voltage of 1.0 V had the surface pores of 168 20 nm in diameter. On the other hand, the etching without an applied voltage gave the approximately two-thirds smaller pores. The conductomeric etching would provide a higher degree of freedom for controlling the pore diameter.
20 nm in diameter. On the other hand, the etching without an applied voltage gave the approximately two-thirds smaller pores. The conductomeric etching would provide a higher degree of freedom for controlling the pore diameter.
 , CO
, CO
 and Br
and Br in seawater; Model calculation of
in seawater; Model calculation of  radiolysis of seawater
radiolysis of seawaterHata, Kuniki; Hanawa, Satoshi; Kasahara, Shigeki; Muroya, Yusa*; Katsumura, Yosuke*
Proceedings of 2012 Nuclear Plant Chemistry Conference (NPC 2012) (CD-ROM), 6 Pages, 2012/09
Fukushima Daiichi Power Plants experienced seawater injection as emergent measure for a short period after the accident. As a result of seawater injection, structural materials in the reactors are exposed to unexpected corrosion environment due to Cl as well as radiolytic products by
as well as radiolytic products by  -rays from fuels. In the present study, a model calculation on seawater radiolysis was carried out to estimate concentration of radiolytic products under
-rays from fuels. In the present study, a model calculation on seawater radiolysis was carried out to estimate concentration of radiolytic products under  -irradiation. Radiolytic products, H
-irradiation. Radiolytic products, H , O
, O and H
and H O
O , in seawater monotonically increased with dose. H
, in seawater monotonically increased with dose. H production was especially distinguished, and its yield was 4.4
production was especially distinguished, and its yield was 4.4  10
10 mol J
mol J . As similar result was obtained from the calculation of NaBr solution, the main species which increased these products in seawater was thought to be Br
. As similar result was obtained from the calculation of NaBr solution, the main species which increased these products in seawater was thought to be Br . On the other hand, Cl
. On the other hand, Cl and HCO
and HCO
 in seawater hardly affected the concentrations of these radiolytic products.
in seawater hardly affected the concentrations of these radiolytic products.
Yamashita, Shinichi; Baldacchino, G.*; Maeyama, Takuya*; Taguchi, Mitsumasa; Muroya, Yusa*; Lin, M.*; Kimura, Atsushi; Murakami, Takeshi*; Katsumura, Yosuke
Free Radical Research, 46(7), p.861 - 871, 2012/07
Times Cited Count:26 Percentile:54.39(Biochemistry & Molecular Biology)Radiation-induced reactions in aqueous solutions of a water-soluble coumarin derivative, coumarin-3-carboxyl acid (C3CA), have been investigated by pulse radiolysis with 35-MeV electron beam, final product analysis after  Co
Co 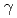 -irradiations, and deterministic model simulations. It was found that C3CA reacts with the hydroxyl radical (
-irradiations, and deterministic model simulations. It was found that C3CA reacts with the hydroxyl radical ( OH) as well as the hydrated electron at nearly diffusion-controlled rate constants: 6.8
OH) as well as the hydrated electron at nearly diffusion-controlled rate constants: 6.8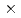 10
10 and 2.1
and 2.1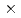 10
10 M
M s
s , respectively. Reactivity of C3CA toward O
, respectively. Reactivity of C3CA toward O

 was not confirmed. Production of a fluorescent molecule 7-hydroxy-coumarin-3-carboxylic acid (7OH-C3CA) was detected by a fluorescence spectrometer coupled with high performance liquid chromatography. Production yields of 7OH-C3CA were in a range from 0.025 to 0.18 (100 eV)
was not confirmed. Production of a fluorescent molecule 7-hydroxy-coumarin-3-carboxylic acid (7OH-C3CA) was detected by a fluorescence spectrometer coupled with high performance liquid chromatography. Production yields of 7OH-C3CA were in a range from 0.025 to 0.18 (100 eV) , depending on irradiation conditions. A variety of the yield with saturating gas, additive, and C3CA concentration implied that there are at least two pathways from scavenging reaction of C3CA toward
, depending on irradiation conditions. A variety of the yield with saturating gas, additive, and C3CA concentration implied that there are at least two pathways from scavenging reaction of C3CA toward  OH to 7OH-C3CA: peroxidation reaction followed by elimination of perhydroxyl radical and disproportionation reaction. A reaction mechanism involving the two pathways was proposed and incorporated into the simulations, showing good explanation of experimentally measured 7OH-C3CA yields with a constant conversion factor from
OH to 7OH-C3CA: peroxidation reaction followed by elimination of perhydroxyl radical and disproportionation reaction. A reaction mechanism involving the two pathways was proposed and incorporated into the simulations, showing good explanation of experimentally measured 7OH-C3CA yields with a constant conversion factor from  OH scavenging to 7OH-C3CA production, 4.7%, unless
OH scavenging to 7OH-C3CA production, 4.7%, unless 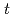 -BuOH is not added.
-BuOH is not added.
Wan, L. K.*; Peng, J.*; Lin, M.; Muroya, Yusa*; Katsumura, Yosuke*; Fu, H. Y.*
Radiation Physics and Chemistry, 81(5), p.524 - 530, 2012/05
Times Cited Count:19 Percentile:77.70(Chemistry, Physical)Rate constants of common crown ethers ((C H
H O)
O) , n=4, 5, 6) and their model compound, 1,4-dioxane(6C2) with some important oxidative radicals,
, n=4, 5, 6) and their model compound, 1,4-dioxane(6C2) with some important oxidative radicals,  OH, SO
OH, SO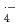 , and NO
, and NO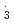 were determined in various aqueous solutions using pulse radiolysis and laser photolysis techniques. The rate constants for 6C2 and crown ethers with
were determined in various aqueous solutions using pulse radiolysis and laser photolysis techniques. The rate constants for 6C2 and crown ethers with  OH and SO
OH and SO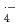 increase with the number of H-atom in the ethers, indicating that H-abstraction is dominant reaction between crown ethers and these two radicals. The presence of cations in solution has negligible effect on the rate constants for
increase with the number of H-atom in the ethers, indicating that H-abstraction is dominant reaction between crown ethers and these two radicals. The presence of cations in solution has negligible effect on the rate constants for  OH and SO
OH and SO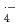 . However, for NO
. However, for NO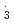 , the rate constants is not in proportional to H-atom number in crown ethers, and the 12-crown-4 is most reactive compared with the others. For the studied crown ethers, the rate constants of these oxidative radicals have the order:
, the rate constants is not in proportional to H-atom number in crown ethers, and the 12-crown-4 is most reactive compared with the others. For the studied crown ethers, the rate constants of these oxidative radicals have the order:  OH
OH  SO
SO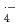
 NO
NO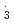 . This is the first report on the kinetic behavior of crown ethers with NO
. This is the first report on the kinetic behavior of crown ethers with NO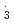 , and it would be helpful for the understanding of stability of crown ethers in the nuclear fuel reprocessing.
, and it would be helpful for the understanding of stability of crown ethers in the nuclear fuel reprocessing.
Lin, M.; Katsumura, Yosuke*; Muroya, Yusa*
Hoshasen Kagaku (Internet), (93), p.3 - 13, 2012/03
The state-of-the-art in the field of water radiolysis at elevated temperatures and supercritical water are reviewed, with the emphasis on the temperature and density effects on the radiolytic yields of water decomposition products, the reaction rate constants and the spectral properties of hydrated electron. The latest studies on supercritical water by picosecond pulse radiolysis as well as some main results about pulse radiolysis of alcohols at elevated temperatures are also introduced.
Fu, H. Y.*; Lin, M.; Muroya, Yusa*; Katsumura, Yosuke*
Research on Chemical Intermediates, 38(1), p.135 - 145, 2012/01
Times Cited Count:2 Percentile:2.69(Chemistry, Multidisciplinary)In the present work, pulse radiolysis was used to demonstrate the oxidizing nature of various intermediates of  -AcMetNH
-AcMetNH and
and  -AcMetOMe via the oxidation of tryptophan (Trp) in a free aqueous solution. The reaction was examined at different pH levels. Electron transfer processes involving Trp and the sulfur-containing three-electron-bonded intermediates were investigated. The results revealed that Trp-Met pairs can form very favorable redox couples in biological systems, which is consistent with earlier reports of more efficient and rapid intramolecular charge transfers in synthetic as well as natural peptides containing methionine (Met).
-AcMetOMe via the oxidation of tryptophan (Trp) in a free aqueous solution. The reaction was examined at different pH levels. Electron transfer processes involving Trp and the sulfur-containing three-electron-bonded intermediates were investigated. The results revealed that Trp-Met pairs can form very favorable redox couples in biological systems, which is consistent with earlier reports of more efficient and rapid intramolecular charge transfers in synthetic as well as natural peptides containing methionine (Met).
Maeyama, Takuya*; Yamashita, Shinichi; Taguchi, Mitsumasa; Baldacchino, G.*; Sihver, L.*; Murakami, Takeshi*; Katsumura, Yosuke
Radiation Physics and Chemistry, 80(12), p.1352 - 1357, 2011/12
Times Cited Count:14 Percentile:69.81(Chemistry, Physical)Coumari-3-carboxylic acid scavenges OH radical produced in water radiolysis, leading to production of a fluorescence probe at almost constant ratio relative to the amount of the scavenged OH radicals. This was applied in estimation of OH radical yield in water radiolysis especially with therapeutic heavy ions of GeV-class energies, i.e.  C
C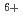 beams of 135, 290 and 400 MeV/u. OH yields upstream of the Bragg peaks decreased with increasing penetration depth of the projectile ions while that downstream suddenly jumped up to near the value for low-LET radiations such as
beams of 135, 290 and 400 MeV/u. OH yields upstream of the Bragg peaks decreased with increasing penetration depth of the projectile ions while that downstream suddenly jumped up to near the value for low-LET radiations such as  -rays. This is due to low-LET secondary fragmentation ions produced during long trajectory of the primary projectile C ion. Quantitative explanation by nuclear fragmentation simulations with PHITS code was attempted and resulted in 15-45% underestimation in the region behind the Bragg peaks, which would be due to the difference in geometries between irradiations of the sample solutions and dosimetry with a small ionization chamber.
-rays. This is due to low-LET secondary fragmentation ions produced during long trajectory of the primary projectile C ion. Quantitative explanation by nuclear fragmentation simulations with PHITS code was attempted and resulted in 15-45% underestimation in the region behind the Bragg peaks, which would be due to the difference in geometries between irradiations of the sample solutions and dosimetry with a small ionization chamber.
Oka, Toshitaka; Yamashita, Shinichi; Midorikawa, Masamichi*; Saiki, Seiichi; Muroya, Yusa*; Kamibayashi, Masato*; Yamashita, Masayuki*; Anzai, Kazunori*; Katsumura, Yosuke*
Analytical Chemistry, 83(24), p.9600 - 9604, 2011/10
Times Cited Count:42 Percentile:78.26(Chemistry, Analytical)Chemical reactions of a novel gauche-type spin trap, G-CYPMPO (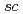 -5-(5,5-dimethyl-2-oxo-1,3,2-dioxaphosphinan-2-yl)-5-methy-1-pyrroline 1-oxide, torsion angle: O1-P1-C6-N1 of 52.8
-5-(5,5-dimethyl-2-oxo-1,3,2-dioxaphosphinan-2-yl)-5-methy-1-pyrroline 1-oxide, torsion angle: O1-P1-C6-N1 of 52.8 ), with reactive oxygen species were examined by pulse radiolysis technique with 35 MeV electron beam and electron spin resonance spectroscopy after
), with reactive oxygen species were examined by pulse radiolysis technique with 35 MeV electron beam and electron spin resonance spectroscopy after  Co
Co  -ray irradiation. The spin trapping reaction rate constants of G-CYPMPO toward hydroxyl radical and hydrated electron were estimated to be (4.2
-ray irradiation. The spin trapping reaction rate constants of G-CYPMPO toward hydroxyl radical and hydrated electron were estimated to be (4.2 0.1)
0.1) 10
10 and (11.8
and (11.8 0.2)
0.2) 10
10 M
M s
s , respectively. Half-lives of spin adducts, hydroxyl radical and perhydroxyl radical adducted G-CYPMPO were estimated to be
, respectively. Half-lives of spin adducts, hydroxyl radical and perhydroxyl radical adducted G-CYPMPO were estimated to be  35 and
35 and  90 min, respectively. A comparison of the results with earlier reports using different radical sources suggests that the purity of the solution and/or the radical generation technique may influence the stability of spin adducts.
90 min, respectively. A comparison of the results with earlier reports using different radical sources suggests that the purity of the solution and/or the radical generation technique may influence the stability of spin adducts.
Fu, H. Y.*; Lin, M.; Katsumura, Yosuke*; Muroya, Yusa*
International Journal of Chemical Kinetics, 43(10), p.590 - 597, 2011/10
Times Cited Count:3 Percentile:5.90(Chemistry, Physical)Free radical scavenging and antioxidant activities of four natural flavonoids, namely silybin, naringenin, naringin and hesperetin, have been studied using nanosecond pulse radiolysis techniques. The kinetics and mechanisms of the reactions of silybin and analogues with various oxidizing radicals have been investigated. Further, the transient species has been assigned and radical scavenging rate constants have also been measured. Moreover, the structure-activity relationships (SAR) between chemical structures of the flavonoids and their radical scavenging activities are further analyzed by theoretical calculation. Combined our previous observation of the fast reparation of DNA damage and efficient DNA protection against radiation damage in vitro, it can be confirmed that test flavonoids are promising molecules to be used for their potential antioxidant properties.
 radical studied by picosecond pulse radiolysis
radical studied by picosecond pulse radiolysisEl Omar, A. K.*; Schmidhammer, U.*; Jeunesse, P.*; Labre, J. P.*; Lin, M.; Muroya, Yusa*; Katsumura, Yosuke*; Pernot, P.*; Mostafavi, M.*
Journal of Physical Chemistry A, 115(44), p.12212 - 12216, 2011/10
Times Cited Count:45 Percentile:81.40(Chemistry, Physical)Picosecond pulse radiolysis measurements using a pulse-probe method are performed to measure directly the time-dependent radiolytic yield of the OH radical in pure water. The time-dependent absorbance of OH
radical in pure water. The time-dependent absorbance of OH radical at 263 nm is deduced from the observed signal by substracting the contribution of the hydated electron and that of the irradiated empty fused silica cell which presents also a transient absoption. The time-dependent radiolytic yield of OH
radical at 263 nm is deduced from the observed signal by substracting the contribution of the hydated electron and that of the irradiated empty fused silica cell which presents also a transient absoption. The time-dependent radiolytic yield of OH is obtained by assuming the yield of the hydrated electron at 20 ps equal to 4.2
is obtained by assuming the yield of the hydrated electron at 20 ps equal to 4.2 10
10 mol/J. The value of the yield of OH
mol/J. The value of the yield of OH radical at 20 ps is found to be (4.80
radical at 20 ps is found to be (4.80 0.12)
0.12) 10
10 mol/J.
mol/J.
 -carrageenan oligomers as plant growth promoter
-carrageenan oligomers as plant growth promoterAbad, L.*; Saiki, Seiichi; Nagasawa, Naotsugu; Kudo, Hisaaki*; Katsumura, Yosuke*; Dela Rosa, A. M.*
Radiation Physics and Chemistry, 80(9), p.977 - 982, 2011/09
Times Cited Count:18 Percentile:77.24(Chemistry, Physical)The optimum molecular weight of irradiated  -carrageenan for the plant growth promoting effect is known to be around 10 kDa, which is obtained by irradiation at doses of 100 kGy in solid and of 2 kGy in 1% aqueous solution. In this study, isolated fraction by membrane filter of irradiated
-carrageenan for the plant growth promoting effect is known to be around 10 kDa, which is obtained by irradiation at doses of 100 kGy in solid and of 2 kGy in 1% aqueous solution. In this study, isolated fraction by membrane filter of irradiated  -carrageenan was analyzed by NMR. The chemical shifts of
-carrageenan was analyzed by NMR. The chemical shifts of  C and
C and  H spectra at the range of 3-10 kDa indicated that the basic functional structure of
H spectra at the range of 3-10 kDa indicated that the basic functional structure of  -carrageenan (alternating D-galactose-4-sulfate and 3,6-anhydro-D-galactose dimer) remains intact. No radiolytic product having carbonyl groups was detected at the range of 3-10 kDa, and moved to less than 3 kDa probably. From these results, it is assumed that the plant growth promoting effect of irradiated
-carrageenan (alternating D-galactose-4-sulfate and 3,6-anhydro-D-galactose dimer) remains intact. No radiolytic product having carbonyl groups was detected at the range of 3-10 kDa, and moved to less than 3 kDa probably. From these results, it is assumed that the plant growth promoting effect of irradiated  -carrageenan depends on the molecular weight, its intact structure, and not on the radiolytic products.
-carrageenan depends on the molecular weight, its intact structure, and not on the radiolytic products.