Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nishimura, Shoichiro*; Torii, Hiroyuki*; Fukao, Yoshinori*; Ito, Takashi; Iwasaki, Masahiko*; Kanda, Sotaro*; Kawagoe, Kiyotomo*; Kawall, D.*; Kawamura, Naritoshi*; Kurosawa, Noriyuki*; et al.
Physical Review A, 104(2), p.L020801_1 - L020801_6, 2021/08
Times Cited Count:13 Percentile:83.13(Optics)Kitamura, Ryo; Bae, S.*; Choi, S.*; Fukao, Yoshinori*; Iinuma, Hiromi*; Ishida, Katsuhiko*; Kawamura, Naritoshi*; Kim, B.*; Kondo, Yasuhiro; Mibe, Tsutomu*; et al.
Physical Review Accelerators and Beams (Internet), 24(3), p.033403_1 - 033403_9, 2021/03
Times Cited Count:1 Percentile:18.91(Physics, Nuclear)A negative muonium ion (Mu ) source using an aluminum foil target was developed as a low-energy muon source. An experiment to produce Mu
) source using an aluminum foil target was developed as a low-energy muon source. An experiment to produce Mu ions was conducted to evaluate the performance of the Mu
ions was conducted to evaluate the performance of the Mu ion source. The measured event rate of Mu
ion source. The measured event rate of Mu ions was
ions was 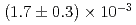 Mu
Mu /s when the event rate of the incident muon beam was
/s when the event rate of the incident muon beam was 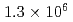 /s. The formation probability, defined as the ratio of the Mu
/s. The formation probability, defined as the ratio of the Mu ions to the incident muons on the Al target, was
ions to the incident muons on the Al target, was 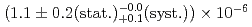 . This Mu
. This Mu ion source boosted the development of the muon accelerator, and the practicality of this low-energy muon source obtained using a relatively simple apparatus was demonstrated.
ion source boosted the development of the muon accelerator, and the practicality of this low-energy muon source obtained using a relatively simple apparatus was demonstrated.
 PdSi
PdSi
Hirschberger, M.*; Nakajima, Taro*; Kriener, M.*; Kurumaji, Takashi*; Spitz, L.*; Gao, S.*; Kikkawa, Akiko*; Yamasaki, Yuichi*; Sagayama, Hajime*; Nakao, Hironori*; et al.
Physical Review B, 101(22), p.220401_1 - 220401_6, 2020/06
Times Cited Count:40 Percentile:91.78(Materials Science, Multidisciplinary)Otani, Masashi*; Fukao, Yoshinori*; Futatsukawa, Kenta*; Kawamura, Naritoshi*; Matoba, Shiro*; Mibe, Tsutomu*; Miyake, Yasuhiro*; Shimomura, Koichiro*; Yamazaki, Takayuki*; Hasegawa, Kazuo; et al.
Journal of Physics; Conference Series, 1350, p.012067_1 - 012067_6, 2019/12
Times Cited Count:2 Percentile:73.22(Physics, Particles & Fields)Negative muonium atom (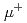 e
e e
e , Mu
, Mu ) has unique features stimulating potential interesting for several scientific fields. Since its discovery in late 1980's in vacuum, it has been discussed that the production efficiency would be improved using a low-work function material. C12A7 was a well-known insulator as a constituent of alumina cement, but was recently confirmed to exhibit electric conductivity by electron doping. The C12A7 electride has relatively low-work function (2.9 eV). In this paper, the negative muonium production measurement with several materials including a C12A7 electride film will be presented. Measured production rate of the Mu
) has unique features stimulating potential interesting for several scientific fields. Since its discovery in late 1980's in vacuum, it has been discussed that the production efficiency would be improved using a low-work function material. C12A7 was a well-known insulator as a constituent of alumina cement, but was recently confirmed to exhibit electric conductivity by electron doping. The C12A7 electride has relatively low-work function (2.9 eV). In this paper, the negative muonium production measurement with several materials including a C12A7 electride film will be presented. Measured production rate of the Mu were 10
were 10 /s for all the Al, electride, and SUS target. Significant enhancement on electride target was not observed, thus it is presumed that the surface condition should be more carefully treated. There was no material dependence of the Mu
/s for all the Al, electride, and SUS target. Significant enhancement on electride target was not observed, thus it is presumed that the surface condition should be more carefully treated. There was no material dependence of the Mu averaged energy: it was 0.2
averaged energy: it was 0.2 0.1keV.
0.1keV.
Strasser, P.*; Abe, Mitsushi*; Aoki, Masaharu*; Choi, S.*; Fukao, Yoshinori*; Higashi, Yoshitaka*; Higuchi, Takashi*; Iinuma, Hiromi*; Ikedo, Yutaka*; Ishida, Katsuhiko*; et al.
EPJ Web of Conferences, 198, p.00003_1 - 00003_8, 2019/01
Times Cited Count:13 Percentile:99.06(Quantum Science & Technology)Otani, Masashi*; Sue, Yuki*; Fukao, Yoshinori*; Futatsukawa, Kenta*; Kawamura, Naritoshi*; Mibe, Tsutomu*; Miyake, Yasuhiro*; Shimomura, Koichiro*; Yamazaki, Takayuki*; Iijima, Toru*; et al.
Journal of Physics; Conference Series, 1067(5), p.052012_1 - 052012_7, 2018/09
Times Cited Count:1 Percentile:47.2(Physics, Particles & Fields)We have measured the muon beam profile after acceleration using a radio frequency quadrupole linac (RFQ). Positive muons are injected to an aluminum degrader and negative muoniums (Mu ) are generated. The generated Mu
) are generated. The generated Mu s are extracted by an electrostatic lens and accelerated to 89 keV by the RFQ. The accelerated Mu
s are extracted by an electrostatic lens and accelerated to 89 keV by the RFQ. The accelerated Mu s are transported to a beam profile monitor (BPM) through a quadrupole magnet pair and a bending magnet. The BPM consists of a micro-channel plate, a phospher screen, and a CCD camera. Measured profile in the vertical direction is consistent to the simulation. This profile measurement is one of milestones for realizing a muon linac for measurement of the muon anomalous magnetic moment at the Japan Proton Accelerator Research Complex.
s are transported to a beam profile monitor (BPM) through a quadrupole magnet pair and a bending magnet. The BPM consists of a micro-channel plate, a phospher screen, and a CCD camera. Measured profile in the vertical direction is consistent to the simulation. This profile measurement is one of milestones for realizing a muon linac for measurement of the muon anomalous magnetic moment at the Japan Proton Accelerator Research Complex.
Kitamura, Ryo*; Otani, Masashi*; Fukao, Yoshinori*; Futatsukawa, Kenta*; Kawamura, Naritoshi*; Mibe, Tsutomu*; Miyake, Yasuhiro*; Yamazaki, Takayuki*; Kondo, Yasuhiro; Hasegawa, Kazuo; et al.
Proceedings of 15th Annual Meeting of Particle Accelerator Society of Japan (Internet), p.239 - 243, 2018/08
Muon acceleration is an important technique in exploring the new frontier of physics. A new measurement of the muon dipole moments is planned in J-PARC using the muon linear accelerator. The low-energy (LE) muon source using the thin metal foil target and beam diagnostic system were developed for the world's first muon acceleration. Negative muonium ions from the thin metal foil target as the LE muon source was successfully observed. Also the beam profile of the LE positive muon was measured by the LE-dedicated beam profile monitor. The muon acceleration test using a Radio-Frequency Quadrupole linac (RFQ) is being prepared as the first step of the muon accelerator development. In this paper, the latest status of the first muon acceleration test is described.
Kitamura, Ryo*; Otani, Masashi*; Fukao, Yoshinori*; Futatsukawa, Kenta*; Kawamura, Naritoshi*; Mibe, Tsutomu*; Miyake, Yasuhiro*; Yamazaki, Takayuki*; Kondo, Yasuhiro; Hasegawa, Kazuo; et al.
Proceedings of 9th International Particle Accelerator Conference (IPAC '18) (Internet), p.1190 - 1193, 2018/06
Muon acceleration using radio-frequency accelerators makes it possible to precisely measure the muon anomalous magnetic moment and the electric dipole moment. The first muon acceleration was demonstrated using a radio-frequency quadrupole (RFQ) linac. A negative muonium ion (Mu ) with less than 2 keV energy was produced from an incident muon with 3 MeV energy using a thin aluminum foil target in order to cool the muon beam for the acceleration, because the designed input energy of the RFQ is 5.6 keV. The Mu
) with less than 2 keV energy was produced from an incident muon with 3 MeV energy using a thin aluminum foil target in order to cool the muon beam for the acceleration, because the designed input energy of the RFQ is 5.6 keV. The Mu was first accelerated to 5.6 keV using an electrostatic accelerator, and was subsequently accelerated to 90 keV using the RFQ. This accelerated Mu
was first accelerated to 5.6 keV using an electrostatic accelerator, and was subsequently accelerated to 90 keV using the RFQ. This accelerated Mu was selected using a diagnostic beam line and was identified based on Time-Of-Flight measurements.
was selected using a diagnostic beam line and was identified based on Time-Of-Flight measurements.
Bae, S.*; Choi, H.*; Choi, S.*; Fukao, Yoshinori*; Futatsukawa, Kenta*; Hasegawa, Kazuo; Iijima, Toru*; Iinuma, Hiromi*; Ishida, Katsuhiko*; Kawamura, Naritoshi*; et al.
Physical Review Accelerators and Beams (Internet), 21(5), p.050101_1 - 050101_6, 2018/05
Times Cited Count:15 Percentile:77.82(Physics, Nuclear)Muons have been accelerated by using a radio-frequency accelerator for the first time. Negative muonium atoms (Mu ), which are bound states of positive muons and two electrons, are generated from through the electron capture process in an aluminum degrader. The generated Mu
), which are bound states of positive muons and two electrons, are generated from through the electron capture process in an aluminum degrader. The generated Mu 's are initially electrostatically accelerated and injected into a radio-frequency quadrupole linac (RFQ). In the RFQ, the Mu
's are initially electrostatically accelerated and injected into a radio-frequency quadrupole linac (RFQ). In the RFQ, the Mu 's are accelerated to 89 keV. The accelerated Mu
's are accelerated to 89 keV. The accelerated Mu 's are identified by momentum measurement and time of flight. This compact muon linac opens the door to various muon accelerator applications including particle physics measurements and the construction of a transmission muon microscope.
's are identified by momentum measurement and time of flight. This compact muon linac opens the door to various muon accelerator applications including particle physics measurements and the construction of a transmission muon microscope.
Kitamura, Ryo*; Otani, Masashi*; Kondo, Yasuhiro; Bae, S.*; Choi, S.*; Fukao, Yoshinori*; Futatsukawa, Kenta*; Hasegawa, Kazuo; Iinuma, Hiromi*; Ishida, Katsuhiko*; et al.
Proceedings of 14th Annual Meeting of Particle Accelerator Society of Japan (Internet), p.100 - 103, 2017/12
Muon acceleration is an important technique in exploring the new frontier of physics. A new measurement of the muon dipole moments is planned in J-PARC using the muon linear accelerator. The low-energy (LE) muon source using the thin metal foil target and beam diagnostic system were developed for the world's first muon acceleration. Negative muonium ions from the thin metal foil target as the LE muon source was successfully observed. Also the beam profile of the LE positive muon was measured by the LE-dedicated beam profile monitor. The muon acceleration test using a Radio-Frequency Quadrupole linac (RFQ) is being prepared as the first step of the muon accelerator development. In this paper, the latest status of the first muon acceleration test is described.
Ueno, Yasuhiro*; Aoki, Masaharu*; Fukao, Yoshinori*; Higashi, Yoshitaka*; Higuchi, Takashi*; Iinuma, Hiromi*; Ikedo, Yutaka*; Ishida, Katsuhiko*; Ito, Takashi; Iwasaki, Masahiko*; et al.
Hyperfine Interactions, 238(1), p.14_1 - 14_6, 2017/11
Times Cited Count:3 Percentile:86.59(Physics, Atomic, Molecular & Chemical)Kitamura, Ryo*; Otani, Masashi*; Fukao, Yoshinori*; Kawamura, Naritoshi*; Mibe, Tsutomu*; Miyake, Yasuhiro*; Shimomura, Koichiro*; Kondo, Yasuhiro; Hasegawa, Kazuo; Bae, S.*; et al.
Proceedings of 8th International Particle Accelerator Conference (IPAC '17) (Internet), p.2311 - 2313, 2017/06
Muon acceleration is an important technique in exploring the new frontier of physics. A new measurement of the muon dipole moments is planned in J-PARC using the muon linear accelerator. The low-energy (LE) muon source using the thin metal foil target and beam diagnostic system were developed for the world's first muon acceleration. Negative muonium ions from the thin metal foil target as the LE muon source was successfully observed. Also the beam profile of the LE positive muon was measured by the LE-dedicated beam profile monitor. The muon acceleration test using a Radio-Frequency Quadrupole linac (RFQ) is being prepared as the first step of the muon accelerator development. In this paper, the latest status of the first muon acceleration test is described.
Kondo, Yasuhiro; Hasegawa, Kazuo; Otani, Masashi*; Mibe, Tsutomu*; Fukao, Yoshinori*; Kawamura, Naritoshi*; Miyake, Yasuhiro*; Shimomura, Koichiro*; Kitamura, Ryo*; Ishida, Katsuhiko*; et al.
Proceedings of 28th International Linear Accelerator Conference (LINAC 2016) (Internet), p.992 - 995, 2017/05
The muon linear accelerator for the muon g-2/EDM experiment in J-PARC is being developed. As the first step of the muon acceleration, the muon acceleration with J-PARC RFQ (Radio-Frequency Quadrupole)-II plans to be demonstrated at H-line of J-PARC MLF. The slow muon will be obtained by the deceleration using the thin metal foil target in the RFQ acceleration test. The intensity of the decelerated muon by the thin metal foil was measured. Based on this result, the beam intensity in the RFQ test at H-line is estimated to be a few /sec. The particle simulation of the RFQ and the following beam diagnostics system is conducted, and it is shown that the emittance measurement at the RFQ exit using the micro-channel plate based beam profile monitor is feasible.
Strasser, P.*; Aoki, Masaharu*; Fukao, Yoshinori*; Higashi, Yoshitaka*; Higuchi, Takashi*; Iinuma, Hiromi*; Ikedo, Yutaka*; Ishida, Katsuhiko*; Ito, Takashi; Iwasaki, Masahiko*; et al.
Hyperfine Interactions, 237(1), p.124_1 - 124_9, 2016/12
Times Cited Count:7 Percentile:90.97(Physics, Atomic, Molecular & Chemical)Kitamura, Ryo*; Otani, Masashi*; Fukao, Yoshinori*; Kawamura, Naritoshi*; Mibe, Tsutomu*; Miyake, Yasuhiro*; Shimomura, Koichiro*; Kondo, Yasuhiro; Hasegawa, Kazuo; Ishida, Katsuhiko*; et al.
Proceedings of 13th Annual Meeting of Particle Accelerator Society of Japan (Internet), p.476 - 479, 2016/11
The muon linear accelerator for the muon g-2/EDM experiment in J-PARC is being developed. As the first step of the muon acceleration, the muon acceleration with J-PARC RFQ (Radio-Frequency Quadrupole)-II plans to be demonstrated at H-line of J-PARC MLF. The slow muon will be obtained by the deceleration using the thin metal foil target in the RFQ acceleration test. The intensity of the decelerated muon by the thin metal foil was measured. Based on this result, the beam intensity in the RFQ test at H-line is estimated to be a few /sec. The particle simulation of the RFQ and the following beam diagnostics system is conducted, and it is shown that the emittance measurement at the RFQ exit using the micro-channel plate based beam profile monitor is feasible.
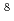 Li
LiIshiyama, Hironobu*; Jeong, S.-C.*; Watanabe, Yutaka*; Hirayama, Yoshikazu*; Imai, Nobuaki*; Jung, H. S.*; Miyatake, Hiroari*; Oyaizu, Mitsuhiro*; Osa, Akihiko; Otokawa, Yoshinori; et al.
Nuclear Instruments and Methods in Physics Research B, 376, p.379 - 381, 2016/06
Times Cited Count:8 Percentile:60.26(Instruments & Instrumentation)Isobe, Kanetsugu; Kawamura, Yoshinori; Iwai, Yasunori; Oyaizu, Makoto; Nakamura, Hirofumi; Suzuki, Takumi; Yamada, Masayuki; Edao, Yuki; Kurata, Rie; Hayashi, Takumi; et al.
Fusion Engineering and Design, 98-99, p.1792 - 1795, 2015/10
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)Activities on Broader Approach (BA) were started in 2007 on the basis of the Agreement between the Government of Japan and the EURATOM. The period of BA activities consist of Phase1 and Phase2 dividing into Phase 2-1 (2010-2011), Phase 2-2 (2012-2013) and Phase 2-3 (2014-2016). Tritium technology was chosen as one of important R&D issues to develop DEMO plant. R&D activities of tritium technology on BA consist of four tasks. Task-1 is to prepare and maintain the tritium handling facility in Rokkasho BA site in Japan. Task 2, 3 and 4 are main R&D activities for tritium and these are focused on: Task-2) Development of tritium accountancy technology, Task-3) Development of basic tritium safety research, Task-4) Tritium durability test. R&D activities of tritium technology in Phase 2-2 were underway successfully and closed in 2013.
Hoshino, Tsuyoshi; Ochiai, Kentaro; Edao, Yuki; Kawamura, Yoshinori
Fusion Science and Technology, 67(2), p.386 - 389, 2015/03
Times Cited Count:13 Percentile:73.22(Nuclear Science & Technology)Demonstration power reactors (DEMOs) require advanced tritium breeders that have high stability at high temperatures. Therefore, the pebble fabrication of Li TiO
TiO with excess Li (Li
with excess Li (Li TiO
TiO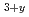 ) as an advanced tritium breeder was carried out. In this study, a preliminary examination of the tritium release properties of advanced tritium breeders was performed. DT neutron irradiation experiments were performed at the fusion neutronics source (FNS) facility in JAEA. The Li
) as an advanced tritium breeder was carried out. In this study, a preliminary examination of the tritium release properties of advanced tritium breeders was performed. DT neutron irradiation experiments were performed at the fusion neutronics source (FNS) facility in JAEA. The Li TiO
TiO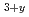 pebbles exhibited good tritium release properties similar to the Li
pebbles exhibited good tritium release properties similar to the Li TiO
TiO pebbles. In particular, the released amount of HT gas for easier tritium handling was higher than that of HTO water.
pebbles. In particular, the released amount of HT gas for easier tritium handling was higher than that of HTO water.
Hayashi, Takumi; Nakamura, Hirofumi; Kawamura, Yoshinori; Iwai, Yasunori; Isobe, Kanetsugu; Yamada, Masayuki; Suzuki, Takumi; Kurata, Rie; Oyaizu, Makoto; Edao, Yuki; et al.
Fusion Science and Technology, 67(2), p.365 - 370, 2015/03
Times Cited Count:1 Percentile:9.74(Nuclear Science & Technology)Edao, Yuki; Kawamura, Yoshinori; Kurata, Rie; Fukada, Satoshi*; Takeishi, Toshiharu*; Hayashi, Takumi; Yamanishi, Toshihiko
Fusion Science and Technology, 67(2), p.320 - 323, 2015/03
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)The present study aims at obtaining fundamental knowledge for tritium transfer behavior and interaction between tritium and paint coated on concrete walls. The amounts of tritium penetration and release in cement paste with epoxy and urethane paint coatings were measured. The tritium penetration amounts were increased with the HTO exposure time. Time to achieve each saturate tritium value was more than 60 days for cement paste coated with epoxy paint and with urethane paint, while cement paste without paint took 2 days to achieve it. Tritium penetration rates were estimated by an analysis of diffusion model. Although their paint coatings were effective for reduction of tritium penetration through the cement paste exposed to HTO for a short period, the amount of tritium trapped in the paints became large for a long time. This work has been performed under the collaboration research between JAEA and Kyushu University.