Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Uno, Yuki; Ouchi, Yasuhiro; Ouchi, Satoshi; Baba, Ryota; Kikuchi, Masanobu; Kawamata, Satoshi
JAEA-Technology 2021-046, 39 Pages, 2023/02
JRR-3 (Japan Research Reactor No.3) is a light water research reactor cooling pool type light water deceleration of low-enriched uranium up to 20MW thermal power. November 1990, begin to operation in modified that we are provided to users as a high neutron flux form reactor facility in various types of irradiation facilities and neutron beam experiment equipment. Currently, JRR-3 has completed the period of facility inspections, which had been extended due to the effects of the Great East Japan Earthquake of March 11, 2011, and has been able to conformity to the New Regulatory Requirements. It has also resumed operation for the first time in about 10 years. FY 2017, overhauled the primary cooling heat exchanger No.1 and No.2 based on a maintenance plan. This is report for take advantage what inspection and maintenance of future about overhaul of the primary cooling system heat exchanger for collect of inspection records and performance.
Kawamura, Sho; Kikuchi, Masanobu; Hosoya, Toshiaki
JAEA-Technology 2021-041, 103 Pages, 2023/02
In response to new regulatory standard for research and test reactor which is enforced December 2013, JRR-3 got license in November 2018 by formulate new design basis ground motion. After that we evaluated for insertion property of control rod using that new design basis ground motion, and that evaluation results were accepted as approval of the design and construction method by Nuclear Regulation Authority. Now, we re-evaluated to insertion property of control rod about neutron absorber and follower fuel element by time history response analysis method. In this report, it shows that new results have sufficiency of margin compared with the past results that are accepted as approval of the design and construction method.
Kikuchi, Masanobu; Kawamura, Sho; Hosoya, Toshiaki
JAEA-Technology 2021-040, 86 Pages, 2023/02
In JRR-3, in response to new regulatory standard for research and test reactor which is enforced December 2013, we established new design basis ground motion for confirming new regulatory standard and carried out seismic evaluations of the appointments, instruments and structures which are installed in JRR-3 by using that earthquake motion. This report shows that the result of evaluations by fatigue strength evaluation, which is more detailed evaluation approach, about Control Rod Drive Mechanism (CRDM) and the CRDM Guide Tube that have gotten the serious result of seismic safety margin by using time history response analysis method. As a result, it was confirmed that CRDM and the CRDM Guide Tube have sufficient seismic safety margin.
Ohshima, Hiroyuki; Morishita, Masaki*; Aizawa, Kosuke; Ando, Masanori; Ashida, Takashi; Chikazawa, Yoshitaka; Doda, Norihiro; Enuma, Yasuhiro; Ezure, Toshiki; Fukano, Yoshitaka; et al.
Sodium-cooled Fast Reactors; JSME Series in Thermal and Nuclear Power Generation, Vol.3, 631 Pages, 2022/07
This book is a collection of the past experience of design, construction, and operation of two reactors, the latest knowledge and technology for SFR designs, and the future prospects of SFR development in Japan. It is intended to provide the perspective and the relevant knowledge to enable readers to become more familiar with SFR technology.
Kitazato, Kohei*; Milliken, R. E.*; Iwata, Takahiro*; Abe, Masanao*; Otake, Makiko*; Matsuura, Shuji*; Takagi, Yasuhiko*; Nakamura, Tomoki*; Hiroi, Takahiro*; Matsuoka, Moe*; et al.
Nature Astronomy (Internet), 5(3), p.246 - 250, 2021/03
Times Cited Count:43 Percentile:96.93(Astronomy & Astrophysics)Here we report observations of Ryugu's subsurface material by the Near-Infrared Spectrometer (NIRS3) on the Hayabusa2 spacecraft. Reflectance spectra of excavated material exhibit a hydroxyl (OH) absorption feature that is slightly stronger and peak-shifted compared with that observed for the surface, indicating that space weathering and/or radiative heating have caused subtle spectral changes in the uppermost surface. However, the strength and shape of the OH feature still suggests that the subsurface material experienced heating above 300  C, similar to the surface. In contrast, thermophysical modeling indicates that radiative heating does not increase the temperature above 200
C, similar to the surface. In contrast, thermophysical modeling indicates that radiative heating does not increase the temperature above 200  C at the estimated excavation depth of 1 m, even if the semimajor axis is reduced to 0.344 au. This supports the hypothesis that primary thermal alteration occurred due to radiogenic and/or impact heating on Ryugu's parent body.
C at the estimated excavation depth of 1 m, even if the semimajor axis is reduced to 0.344 au. This supports the hypothesis that primary thermal alteration occurred due to radiogenic and/or impact heating on Ryugu's parent body.
 -ray irradiation in HNO
-ray irradiation in HNO media
mediaNogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 296(1), p.423 - 427, 2013/04
Times Cited Count:5 Percentile:38.62(Chemistry, Analytical)Stability of polyvinylpolypyrrolidone (PVPP), a resin with adsorption selectivity to U(VI) in nitric acid media, against  -ray irradiation has been examined using HNO
-ray irradiation has been examined using HNO solutions of various concentrations. As the result, no clear decrease in the capacity was observed for any samples. Or rather, it was found that the capacity increased by approximately 50% for the PVPP slurry irradiated in 6 M HNO
solutions of various concentrations. As the result, no clear decrease in the capacity was observed for any samples. Or rather, it was found that the capacity increased by approximately 50% for the PVPP slurry irradiated in 6 M HNO . The infrared spectroscopic study indicates that PVPP degrades by
. The infrared spectroscopic study indicates that PVPP degrades by  -ray irradiation in HNO
-ray irradiation in HNO from the cleavage of the pyrrolidone ring by the addition of oxygen atom originating from HNO
from the cleavage of the pyrrolidone ring by the addition of oxygen atom originating from HNO , followed by the formation of chain monoamides with multiple coordinative atoms by the continuous addition of oxygen, finally leading to the generation of primary-amine type anion exchange resin. It is also indicated that all generated functional groups possess adsorptivity to U(VI) in 3 M HNO
, followed by the formation of chain monoamides with multiple coordinative atoms by the continuous addition of oxygen, finally leading to the generation of primary-amine type anion exchange resin. It is also indicated that all generated functional groups possess adsorptivity to U(VI) in 3 M HNO .
.
 -ray irradiation
-ray irradiationNogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Science China; Chemistry, 55(9), p.1739 - 1745, 2012/09
Times Cited Count:4 Percentile:19.69(Chemistry, Multidisciplinary)Stability of N-alkylated pyrrolidone derivatives (NRPs) against radiation was examined by irradiation tests with  Co
Co  -ray. We have been developed a novel reprocessing system using NRPs which have precipitation ability to haxa- and tetravalent actinides in nitric acid media. Degradation rates of NRPs are evaluated by irradiation in 3M nitric acid solutions and mechanism of degradation are discussed in the present paper.
-ray. We have been developed a novel reprocessing system using NRPs which have precipitation ability to haxa- and tetravalent actinides in nitric acid media. Degradation rates of NRPs are evaluated by irradiation in 3M nitric acid solutions and mechanism of degradation are discussed in the present paper.
Ikeda, Yasuhisa*; Kawasaki, Takeshi*; Harada, Masayuki*; Nogami, Masanobu*; Kawata, Yoshihisa*; Kim, S.-Y.*; Morita, Yasuji; Chikazawa, Takahiro*; Someya, Hiroshi*; Kikuchi, Toshiaki*
Proceedings of International Conference on Toward and Over the Fukushima Daiichi Accident (GLOBAL 2011) (CD-ROM), 5 Pages, 2011/12
An advanced reprocessing system for spent FBR fuels based on two precipitation processes using pyrrolidone derivatives as precipitants has been developed. Experimental results of precipitation behavior of U, Pu and other elements, the heat- and radiation-resistance of precipitants, the thermal decomposition properties of precipitates showed that N-n-butyl-2-pyrrolidone and N-neopentyl-2-pyrrolidone are the appropriate precipitants for the first and second precipitation steps, respectively. From the engineering investigation, We confirmed that the precipitation and the filtration can be done efficiently using the engineering scale equipment and that the fuel pellets are directly prepared by the calcination of the precipitates. On the basis of these results, we evaluated that the proposed system is expected to be one of candidates of the future reprocessing systems for spent FBR fuels.
Nogami, Masanobu*; Harada, Masayuki*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Progress in Nuclear Energy, 53(7), p.948 - 951, 2011/09
Times Cited Count:3 Percentile:26.02(Nuclear Science & Technology)The precipitation ability of 1,3-dimethyl-2-imidazolidone (DMI) to U(VI) and U(IV) was examined using nitric acid solutions. While DMI precipitated U(VI) from 3 M nitric acid, no precipitate was observed in the solution containing 0.15 M U(IV) and 3 M nitric acid by adding DMI at the ratio of [DMI]/[U(IV)]=5. This indicates that the selectivity of DMI to U(VI) than U(IV). In spite of the excellent selectivity to U(VI), DMI has a disadvantage on the stability in nitric acid, because gradual acid hydrolysis of DMI is inevitable due to the nature of the chemical structure. Experiments on the stability of DMI in  -ray irradiation and heating in nitric acid solutions showed that the stability is strongly affected by the concentration of nitric acid and that DMI may be applicable in nitric acid solutions up to ca. 2 M.
-ray irradiation and heating in nitric acid solutions showed that the stability is strongly affected by the concentration of nitric acid and that DMI may be applicable in nitric acid solutions up to ca. 2 M.
Washiya, Tadahiro; Tayama, Toshimitsu; Nakamura, Kazuhito*; Yano, Kimihiko; Shibata, Atsuhiro; Nomura, Kazunori; Chikazawa, Takahiro*; Nagata, Masanobu*; Kikuchi, Toshiaki*
Journal of Power and Energy Systems (Internet), 4(1), p.191 - 201, 2010/02
Japan Atomic Energy Agency (JAEA) and Mitsubishi Materials Corporation (MMC) are developing the crystallization process for elemental technology of FBR fuel reprocessing. The uranium (U) crystallization process is a key technology for New Extraction System for TRU Recovery (NEXT) process that was evaluated as the most promising process for future FBR reprocessing. We had developed an innovative crystallizer and fabricated an engineering-scale crystallizer and have carried out continuous operation test to investigate the stability of the equipment at steady and non-steady state conditions by using depleted uranium. As for simulating typical failure events in the crystallizer, crystal accumulation and crystal blockage were occurred intentionally, and monitoring method and resume procedure were tried and selected in this work.
Nogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 283(2), p.541 - 546, 2010/02
Times Cited Count:20 Percentile:78.77(Chemistry, Analytical)Adsorptivity of polyvinylpolypyrrolidone (PVPP) to various metal ions was examined as a part of the development of resins with selectivity to U(VI) in nitric acid media. It was found that PVPP has a strong adsorptivity to U(VI) in wide concentration range of nitric acid. The mechanism of U(VI) adsorption by PVPP was discussed by results of Scatchard plot analysis and infrared spectroscopic analysis. Furthermore, it was found that fission productions except for Re(VII) as the simulant of Tc(VII) and Pd(II) are not adsorbed on to PVPP and that Pd(II) and Re(VII) species are weakly adsorbed in the lower concentration ranges of nitric acid, where the adsorption rates of Pd(II) are extremely slower than those of U(VI). These results indicate that U(VI) can be separated from other metal ions by PVPP.
 -ray irradiation
-ray irradiationNogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Kawata, Yoshihisa; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
JAEA-Review 2009-041, JAEA Takasaki Annual Report 2008, P. 25, 2009/12
As a part of the development of a novel reprocessing system for spent FBR fuels based on the precipitation method, influence of concentrations of HNO on the stability by
on the stability by  -ray irradiation was examined for
-ray irradiation was examined for  -
- -butyl-2-pyrrolidone (NBP), a candidate precipitant for the first precipitation step for selectively precipitating U(VI). The residual ratios of the samples for HNO
-butyl-2-pyrrolidone (NBP), a candidate precipitant for the first precipitation step for selectively precipitating U(VI). The residual ratios of the samples for HNO solutions up to 3 M were found to be decreased identically, where ca. 20% of NBP was degraded after the irradiation of 1 MGy. It was found that the degradation of the samples of 6 M HNO
solutions up to 3 M were found to be decreased identically, where ca. 20% of NBP was degraded after the irradiation of 1 MGy. It was found that the degradation of the samples of 6 M HNO is more distinguished, where ca. 30% was degraded after the irradiation of 0.1 MGy. As the result of the investigation of the degradation mechanism of NBP, it was revealed that the degradation started from the cleavage of the pyrrolidone ring of NBP by the addition of oxygen atom, followed by the formation of chain monoamides and C4 compounds by the continuous addition of oxygen, leading to the generation of oxalic acid.
is more distinguished, where ca. 30% was degraded after the irradiation of 0.1 MGy. As the result of the investigation of the degradation mechanism of NBP, it was revealed that the degradation started from the cleavage of the pyrrolidone ring of NBP by the addition of oxygen atom, followed by the formation of chain monoamides and C4 compounds by the continuous addition of oxygen, leading to the generation of oxalic acid.
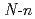 -butyl-2-pyrrolidone,
-butyl-2-pyrrolidone,  -cyclohexylmethyl-2-pyrrolidone, and 1,3-dimethyl-2-imidazolidone
-cyclohexylmethyl-2-pyrrolidone, and 1,3-dimethyl-2-imidazolidoneKoshino, Nobuyoshi*; Harada, Masayuki*; Nogami, Masanobu*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Inorganica Chimica Acta, 358(6), p.1857 - 1864, 2005/03
Times Cited Count:53 Percentile:88.15(Chemistry, Inorganic & Nuclear)Structural analyses of UO (NO
(NO )
) L
L [L=
[L=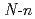 -butyl-2-pyrrolidone, N-cyclohexylmethyl-2-pyrrolidone, and 1,3-dimethyl-2-imidazolidone] have been carried out using X-ray diffraction method. These uranyl complexes were found to have a hexagonal bipyramidal structure. The bond distances of U=O and U-O (ligand), and bond angles of U-O-C(carbonyl) are determined. In uranyl nitrate complexes with cyclic amides such as 2-pyrrolidone, urea, and caprolactam derivatives, a linear correlation was found to hold between U-O (ligand) bond distances and U-O-C(carbonyl) bond angles. Vibrational frequencies of UO
-butyl-2-pyrrolidone, N-cyclohexylmethyl-2-pyrrolidone, and 1,3-dimethyl-2-imidazolidone] have been carried out using X-ray diffraction method. These uranyl complexes were found to have a hexagonal bipyramidal structure. The bond distances of U=O and U-O (ligand), and bond angles of U-O-C(carbonyl) are determined. In uranyl nitrate complexes with cyclic amides such as 2-pyrrolidone, urea, and caprolactam derivatives, a linear correlation was found to hold between U-O (ligand) bond distances and U-O-C(carbonyl) bond angles. Vibrational frequencies of UO (NO
(NO )
) L
L have also been measured by IR and Raman spectrophotometers. Using relationships between vibrational frequencies of O=U=O bonds and donor numbers (DNs) of ligands, donicities of N-substituted-2-pyrrolidones were determined.
have also been measured by IR and Raman spectrophotometers. Using relationships between vibrational frequencies of O=U=O bonds and donor numbers (DNs) of ligands, donicities of N-substituted-2-pyrrolidones were determined.
Noda, Kyoko*; Takao, Koichiro*; Sugiyama, Yuichi*; Harada, Masayuki*; Nogami, Masanobu*; Maruyama, Koichi*; Takahashi, Hiroaki*; Kim, S.-Y.; Sato, Makoto; Mineo, Hideaki; et al.
no journal, ,
We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives. In previous investigation, N-cyclohexyl-2-pyrrolidone (NCP) is used as a precipitant, which is able to precipitate selectively UO
 ions in HNO
ions in HNO solution, and a process consisting of two separation steps; selective U precipitation step and U-Pu co-precipitation step, was developed. In order to make the process more effective and more economical, we are now studying precipitation of U and Pu with other pyrrolidone derivatives. The outline of the study and main results obtained until now are shown in this presentation.
solution, and a process consisting of two separation steps; selective U precipitation step and U-Pu co-precipitation step, was developed. In order to make the process more effective and more economical, we are now studying precipitation of U and Pu with other pyrrolidone derivatives. The outline of the study and main results obtained until now are shown in this presentation.
Washiya, Tadahiro; Chikazawa, Takahiro*; Nagata, Masanobu*; Kikuchi, Toshiaki*; Hirasawa, Izumi*
no journal, ,
no abstracts in English
Nogami, Masanobu*; Kawasaki, Takeshi*; Takao, Koichiro*; Noda, Kyoko*; Sugiyama, Yuichi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Morita, Yasuji; Chikazawa, Takahiro*; Kikuchi, Toshiaki*; et al.
no journal, ,
A reprocessing system for spent FBR fuels based on the two precipitation processes has been proposed. In this system, first only U(VI) species are precipitated using pyrrolidone derivatives (NRP) with low hydrophobicity and donicity, and secondly residual U(VI) and Pu(IV, VI) are precipitated simultaneously using pyrrolidone derivative with high precipitation ability. In order to develop such a reprocessing method, behavior of uranyl ions in HNO has been studied using various novel NRP. In this presentation, recent advances on precipitation behavior and of uranyl ions and calcination of U(VI)-NRP will be introduced.
has been studied using various novel NRP. In this presentation, recent advances on precipitation behavior and of uranyl ions and calcination of U(VI)-NRP will be introduced.
Kim, S.-Y.; Kawata, Yoshihisa; Morita, Yasuji; Nogami, Masanobu*; Harada, Masayuki*; Ikeda, Yasuhisa*; Kikuchi, Toshiaki*; Nishimura, Kenji*
no journal, ,
A reprocessing system for spent FBR fuels based on the two precipitation processes has been proposed. In this system, first only U(VI) species are precipitated using pyrrolidone derivatives (NRP) with low hydrophobicity and donicity, and secondly residual U(VI) and Pu(IV, VI) are precipitated simultaneously using pyrrolidone derivative with high precipitation ability. It is important to control precipitation of Pu(IV), and recent advances on precipitation behavior of Pu(IV, VI) by using novel pyrrolidone derivatives will be introduced in this presentation. Precipitation tests using mixed solutions of U(VI) and Pu(IV) showed that N-n-butyl-2-pyrrolidone (NBP) is the most appropriate precipitant for the first precipitation step and N-neopenthyl-2-pyrrolidone (NNpP) is the most appropriate precipitant for the second step.
Oyama, Koichi; Katsurai, Kiyomichi; Kondo, Yoshikazu; Washiya, Tadahiro; Myochin, Munetaka; Nagata, Masanobu*; Horiuchi, Nobutake*; Chikazawa, Takahiro*; Kikuchi, Toshiaki*
no journal, ,
no abstracts in English
Shibahara, Takahiro*; Nagata, Masanobu*; Chikazawa, Takahiro*; Kikuchi, Toshiaki*; Morita, Yasuji; Ikeda, Yasuhisa*
no journal, ,
Thermal decomposition of precipitants of U(VI) with pyrrolidone derivatives was investigated for the development of an advanced reprocessing system for spent FBR fuels based only on precipitation method using the pyrrolidone derivatives. It was found that the thermal treatment of U(VI) precipitates at constant temperature of 150 - 170  C before the decomposition of the precipitate at high temperature contribute to the production of the U oxides with low impurities and the recovery of the with pyrrolidone derivatives.
C before the decomposition of the precipitate at high temperature contribute to the production of the U oxides with low impurities and the recovery of the with pyrrolidone derivatives.
Kawasaki, Takeshi*; Nogami, Masanobu*; Sugiyama, Yuichi*; Harada, Masayuki*; Ikeda, Yasuhisa*; Morita, Yasuji; Kikuchi, Toshiaki*
no journal, ,
Recovery of the precipitant by evaporation of the precipitates of U(VI) with pyrrolidone derivatives was investigated for the development of an advanced reprocessing system for spent FBR fuels based only on precipitation method. It was found that the U(VI) precipitate with N-n-butyl-2-pyrrolidone (NBP) melted at about 100 C and the bubble formation was observed at 140 - 150
C and the bubble formation was observed at 140 - 150 C. Most of the recovered materials by cooling the evaporated fraction was NBP, and therefore, it is expected to recover the precipitant without degradation by this methods.
C. Most of the recovered materials by cooling the evaporated fraction was NBP, and therefore, it is expected to recover the precipitant without degradation by this methods.