Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 O/H
O/H O vapor generator for contrast-variation neutron scattering
O vapor generator for contrast-variation neutron scatteringArima-Osonoi, Hiroshi*; Takata, Shinichi; Kasai, Satoshi*; Ouchi, Keiichi*; Morikawa, Toshiaki*; Miyata, Noboru*; Miyazaki, Tsukasa*; Aoki, Hiroyuki; Iwase, Hiroki*; Hiroi, Kosuke; et al.
Journal of Applied Crystallography, 56(6), p.1802 - 1812, 2023/12
Times Cited Count:0 Percentile:0.02(Chemistry, Multidisciplinary)Kawamura, Sho; Kikuchi, Masanobu; Hosoya, Toshiaki
JAEA-Technology 2021-041, 103 Pages, 2023/02
In response to new regulatory standard for research and test reactor which is enforced December 2013, JRR-3 got license in November 2018 by formulate new design basis ground motion. After that we evaluated for insertion property of control rod using that new design basis ground motion, and that evaluation results were accepted as approval of the design and construction method by Nuclear Regulation Authority. Now, we re-evaluated to insertion property of control rod about neutron absorber and follower fuel element by time history response analysis method. In this report, it shows that new results have sufficiency of margin compared with the past results that are accepted as approval of the design and construction method.
Kikuchi, Masanobu; Kawamura, Sho; Hosoya, Toshiaki
JAEA-Technology 2021-040, 86 Pages, 2023/02
In JRR-3, in response to new regulatory standard for research and test reactor which is enforced December 2013, we established new design basis ground motion for confirming new regulatory standard and carried out seismic evaluations of the appointments, instruments and structures which are installed in JRR-3 by using that earthquake motion. This report shows that the result of evaluations by fatigue strength evaluation, which is more detailed evaluation approach, about Control Rod Drive Mechanism (CRDM) and the CRDM Guide Tube that have gotten the serious result of seismic safety margin by using time history response analysis method. As a result, it was confirmed that CRDM and the CRDM Guide Tube have sufficient seismic safety margin.
Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Nakajima, Kenji; Kawamura, Seiko; Kikuchi, Tatsuya; Fujiwara, Satoru
Biochemistry and Biophysics Reports (Internet), 6, p.220 - 225, 2016/07
Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Nakajima, Kenji; Kawamura, Seiko; Kikuchi, Tatsuya; Fujiwara, Satoru
Biochemical and Biophysical Research Communications, 459(3), p.493 - 497, 2015/04
Times Cited Count:4 Percentile:12.91(Biochemistry & Molecular Biology) -ray irradiation in HNO
-ray irradiation in HNO media
mediaNogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 296(1), p.423 - 427, 2013/04
Times Cited Count:5 Percentile:38.62(Chemistry, Analytical)Stability of polyvinylpolypyrrolidone (PVPP), a resin with adsorption selectivity to U(VI) in nitric acid media, against  -ray irradiation has been examined using HNO
-ray irradiation has been examined using HNO solutions of various concentrations. As the result, no clear decrease in the capacity was observed for any samples. Or rather, it was found that the capacity increased by approximately 50% for the PVPP slurry irradiated in 6 M HNO
solutions of various concentrations. As the result, no clear decrease in the capacity was observed for any samples. Or rather, it was found that the capacity increased by approximately 50% for the PVPP slurry irradiated in 6 M HNO . The infrared spectroscopic study indicates that PVPP degrades by
. The infrared spectroscopic study indicates that PVPP degrades by  -ray irradiation in HNO
-ray irradiation in HNO from the cleavage of the pyrrolidone ring by the addition of oxygen atom originating from HNO
from the cleavage of the pyrrolidone ring by the addition of oxygen atom originating from HNO , followed by the formation of chain monoamides with multiple coordinative atoms by the continuous addition of oxygen, finally leading to the generation of primary-amine type anion exchange resin. It is also indicated that all generated functional groups possess adsorptivity to U(VI) in 3 M HNO
, followed by the formation of chain monoamides with multiple coordinative atoms by the continuous addition of oxygen, finally leading to the generation of primary-amine type anion exchange resin. It is also indicated that all generated functional groups possess adsorptivity to U(VI) in 3 M HNO .
.
 -ray irradiation
-ray irradiationNogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Science China; Chemistry, 55(9), p.1739 - 1745, 2012/09
Times Cited Count:4 Percentile:19.69(Chemistry, Multidisciplinary)Stability of N-alkylated pyrrolidone derivatives (NRPs) against radiation was examined by irradiation tests with  Co
Co  -ray. We have been developed a novel reprocessing system using NRPs which have precipitation ability to haxa- and tetravalent actinides in nitric acid media. Degradation rates of NRPs are evaluated by irradiation in 3M nitric acid solutions and mechanism of degradation are discussed in the present paper.
-ray. We have been developed a novel reprocessing system using NRPs which have precipitation ability to haxa- and tetravalent actinides in nitric acid media. Degradation rates of NRPs are evaluated by irradiation in 3M nitric acid solutions and mechanism of degradation are discussed in the present paper.
Ikeda, Yasuhisa*; Kawasaki, Takeshi*; Harada, Masayuki*; Nogami, Masanobu*; Kawata, Yoshihisa*; Kim, S.-Y.*; Morita, Yasuji; Chikazawa, Takahiro*; Someya, Hiroshi*; Kikuchi, Toshiaki*
Proceedings of International Conference on Toward and Over the Fukushima Daiichi Accident (GLOBAL 2011) (CD-ROM), 5 Pages, 2011/12
An advanced reprocessing system for spent FBR fuels based on two precipitation processes using pyrrolidone derivatives as precipitants has been developed. Experimental results of precipitation behavior of U, Pu and other elements, the heat- and radiation-resistance of precipitants, the thermal decomposition properties of precipitates showed that N-n-butyl-2-pyrrolidone and N-neopentyl-2-pyrrolidone are the appropriate precipitants for the first and second precipitation steps, respectively. From the engineering investigation, We confirmed that the precipitation and the filtration can be done efficiently using the engineering scale equipment and that the fuel pellets are directly prepared by the calcination of the precipitates. On the basis of these results, we evaluated that the proposed system is expected to be one of candidates of the future reprocessing systems for spent FBR fuels.
Nogami, Masanobu*; Harada, Masayuki*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Kawata, Yoshihisa*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Progress in Nuclear Energy, 53(7), p.948 - 951, 2011/09
Times Cited Count:3 Percentile:26.02(Nuclear Science & Technology)The precipitation ability of 1,3-dimethyl-2-imidazolidone (DMI) to U(VI) and U(IV) was examined using nitric acid solutions. While DMI precipitated U(VI) from 3 M nitric acid, no precipitate was observed in the solution containing 0.15 M U(IV) and 3 M nitric acid by adding DMI at the ratio of [DMI]/[U(IV)]=5. This indicates that the selectivity of DMI to U(VI) than U(IV). In spite of the excellent selectivity to U(VI), DMI has a disadvantage on the stability in nitric acid, because gradual acid hydrolysis of DMI is inevitable due to the nature of the chemical structure. Experiments on the stability of DMI in  -ray irradiation and heating in nitric acid solutions showed that the stability is strongly affected by the concentration of nitric acid and that DMI may be applicable in nitric acid solutions up to ca. 2 M.
-ray irradiation and heating in nitric acid solutions showed that the stability is strongly affected by the concentration of nitric acid and that DMI may be applicable in nitric acid solutions up to ca. 2 M.
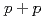 collisions at
collisions at 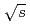 = 200 and 62.4 GeV
= 200 and 62.4 GeVAdare, A.*; Afanasiev, S.*; Aidala, C.*; Ajitanand, N. N.*; Akiba, Yasuyuki*; Al-Bataineh, H.*; Alexander, J.*; Aoki, Kazuya*; Aphecetche, L.*; Armendariz, R.*; et al.
Physical Review C, 83(6), p.064903_1 - 064903_29, 2011/06
Times Cited Count:184 Percentile:99.44(Physics, Nuclear)Transverse momentum distributions and yields for 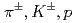 , and
, and 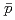 in
in 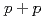 collisions at
collisions at 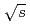 = 200 and 62.4 GeV at midrapidity are measured by the PHENIX experiment at the RHIC. We present the inverse slope parameter, mean transverse momentum, and yield per unit rapidity at each energy, and compare them to other measurements at different
= 200 and 62.4 GeV at midrapidity are measured by the PHENIX experiment at the RHIC. We present the inverse slope parameter, mean transverse momentum, and yield per unit rapidity at each energy, and compare them to other measurements at different 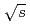 collisions. We also present the scaling properties such as
collisions. We also present the scaling properties such as 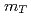 and
and 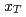 scaling and discuss the mechanism of the particle production in
scaling and discuss the mechanism of the particle production in 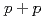 collisions. The measured spectra are compared to next-to-leading order perturbative QCD calculations.
collisions. The measured spectra are compared to next-to-leading order perturbative QCD calculations.
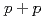 and Au+Au collisions at
and Au+Au collisions at 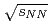 = 200 GeV
= 200 GeVAdare, A.*; Afanasiev, S.*; Aidala, C.*; Ajitanand, N. N.*; Akiba, Yasuyuki*; Al-Bataineh, H.*; Alexander, J.*; Aoki, Kazuya*; Aphecetche, L.*; Aramaki, Y.*; et al.
Physical Review C, 83(4), p.044912_1 - 044912_16, 2011/04
Times Cited Count:8 Percentile:49.7(Physics, Nuclear)Measurements of electrons from the decay of open-heavy-flavor mesons have shown that the yields are suppressed in Au+Au collisions compared to expectations from binary-scaled 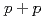 collisions. Here we extend these studies to two particle correlations where one particle is an electron from the decay of a heavy flavor meson and the other is a charged hadron from either the decay of the heavy meson or from jet fragmentation. These measurements provide more detailed information about the interaction between heavy quarks and the quark-gluon matter. We find the away-side-jet shape and yield to be modified in Au+Au collisions compared to
collisions. Here we extend these studies to two particle correlations where one particle is an electron from the decay of a heavy flavor meson and the other is a charged hadron from either the decay of the heavy meson or from jet fragmentation. These measurements provide more detailed information about the interaction between heavy quarks and the quark-gluon matter. We find the away-side-jet shape and yield to be modified in Au+Au collisions compared to 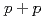 collisions.
collisions.
Washiya, Tadahiro; Tayama, Toshimitsu; Nakamura, Kazuhito*; Yano, Kimihiko; Shibata, Atsuhiro; Nomura, Kazunori; Chikazawa, Takahiro*; Nagata, Masanobu*; Kikuchi, Toshiaki*
Journal of Power and Energy Systems (Internet), 4(1), p.191 - 201, 2010/02
Japan Atomic Energy Agency (JAEA) and Mitsubishi Materials Corporation (MMC) are developing the crystallization process for elemental technology of FBR fuel reprocessing. The uranium (U) crystallization process is a key technology for New Extraction System for TRU Recovery (NEXT) process that was evaluated as the most promising process for future FBR reprocessing. We had developed an innovative crystallizer and fabricated an engineering-scale crystallizer and have carried out continuous operation test to investigate the stability of the equipment at steady and non-steady state conditions by using depleted uranium. As for simulating typical failure events in the crystallizer, crystal accumulation and crystal blockage were occurred intentionally, and monitoring method and resume procedure were tried and selected in this work.
Nogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 283(2), p.541 - 546, 2010/02
Times Cited Count:20 Percentile:78.77(Chemistry, Analytical)Adsorptivity of polyvinylpolypyrrolidone (PVPP) to various metal ions was examined as a part of the development of resins with selectivity to U(VI) in nitric acid media. It was found that PVPP has a strong adsorptivity to U(VI) in wide concentration range of nitric acid. The mechanism of U(VI) adsorption by PVPP was discussed by results of Scatchard plot analysis and infrared spectroscopic analysis. Furthermore, it was found that fission productions except for Re(VII) as the simulant of Tc(VII) and Pd(II) are not adsorbed on to PVPP and that Pd(II) and Re(VII) species are weakly adsorbed in the lower concentration ranges of nitric acid, where the adsorption rates of Pd(II) are extremely slower than those of U(VI). These results indicate that U(VI) can be separated from other metal ions by PVPP.
 -ray irradiation
-ray irradiationNogami, Masanobu*; Sugiyama, Yuichi*; Kawasaki, Takeshi*; Harada, Masayuki*; Kawata, Yoshihisa; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
JAEA-Review 2009-041, JAEA Takasaki Annual Report 2008, P. 25, 2009/12
As a part of the development of a novel reprocessing system for spent FBR fuels based on the precipitation method, influence of concentrations of HNO on the stability by
on the stability by  -ray irradiation was examined for
-ray irradiation was examined for  -
- -butyl-2-pyrrolidone (NBP), a candidate precipitant for the first precipitation step for selectively precipitating U(VI). The residual ratios of the samples for HNO
-butyl-2-pyrrolidone (NBP), a candidate precipitant for the first precipitation step for selectively precipitating U(VI). The residual ratios of the samples for HNO solutions up to 3 M were found to be decreased identically, where ca. 20% of NBP was degraded after the irradiation of 1 MGy. It was found that the degradation of the samples of 6 M HNO
solutions up to 3 M were found to be decreased identically, where ca. 20% of NBP was degraded after the irradiation of 1 MGy. It was found that the degradation of the samples of 6 M HNO is more distinguished, where ca. 30% was degraded after the irradiation of 0.1 MGy. As the result of the investigation of the degradation mechanism of NBP, it was revealed that the degradation started from the cleavage of the pyrrolidone ring of NBP by the addition of oxygen atom, followed by the formation of chain monoamides and C4 compounds by the continuous addition of oxygen, leading to the generation of oxalic acid.
is more distinguished, where ca. 30% was degraded after the irradiation of 0.1 MGy. As the result of the investigation of the degradation mechanism of NBP, it was revealed that the degradation started from the cleavage of the pyrrolidone ring of NBP by the addition of oxygen atom, followed by the formation of chain monoamides and C4 compounds by the continuous addition of oxygen, leading to the generation of oxalic acid.
Shibata, Atsuhiro; Yano, Kimihiko; Kamiya, Masayoshi; Nakamura, Kazuhito; Washiya, Tadahiro; Chikazawa, Takahiro*; Kikuchi, Toshiaki*
Nihon Genshiryoku Gakkai Wabun Rombunshi, 8(3), p.245 - 253, 2009/09
Behavior of Cs in U crystallization process of advanced aqueous reprocessing system was investigated with simulated dissolver solution. Beaker-scale U crystallization experiments were carried out with some simulated dissolver solutions. The results show that possibility of generation of CsNO ,Cs
,Cs UO
UO (NO
(NO )
) or Cs-FP complex salt is small. Precipitation experiments of Cs-U(IV) complex salts were also carried out with nitrate solution of U(IV) and Cs. It was found that Cs-U(IV) complex salt was precipitated in higher acidity than 5 mol/dm
or Cs-FP complex salt is small. Precipitation experiments of Cs-U(IV) complex salts were also carried out with nitrate solution of U(IV) and Cs. It was found that Cs-U(IV) complex salt was precipitated in higher acidity than 5 mol/dm . It is suggested that Cs-Pu(IV) precipitates can be generated in the U crystallization process, under specific solution condition.
. It is suggested that Cs-Pu(IV) precipitates can be generated in the U crystallization process, under specific solution condition.
Yano, Kimihiko; Nakahara, Masaumi; Nakamura, Masahiro; Shibata, Atsuhiro; Nomura, Kazunori; Nakamura, Kazuhito*; Tayama, Toshimitsu; Washiya, Tadahiro; Chikazawa, Takahiro*; Kikuchi, Toshiaki*; et al.
Proceedings of International Conference on Advanced Nuclear Fuel Cycle; Sustainable Options & Industrial Perspectives (Global 2009) (CD-ROM), p.143 - 150, 2009/09
Shibata, Atsuhiro; Kaji, Naoya; Nakahara, Masaumi; Yano, Kimihiko; Tayama, Toshimitsu; Nakamura, Kazuhito; Washiya, Tadahiro; Myochin, Munetaka; Chikazawa, Takahiro*; Kikuchi, Toshiaki*
Proceedings of International Conference on Advanced Nuclear Fuel Cycle; Sustainable Options & Industrial Perspectives (Global 2009) (CD-ROM), p.151 - 157, 2009/09
As a part of FaCT project, Japan Atomic Energy Agency has been developing a U crystallization process for advanced aqueous reprocessing technology in collaboration with Mitsubishi Materials Corporation. We have carried out experimental studies and obtained fundamental data. Continuous operation tests were also carried out by an engineering-scale crystallizer to confirm productivity of the equipment and to investigate non-steady state conditions. The requirements for the U crystallization process in the FaCT project could be achieved except DF of Cs. More detail investigation is under way to settle the process condition without Pu-Cs double salt formation.
Morita, Yasuji; Kim, S.-Y.; Kawata, Yoshihisa; Ikeda, Yasuhisa*; Kikuchi, Toshiaki*
Proceedings of International Conference on Advanced Nuclear Fuel Cycle; Sustainable Options & Industrial Perspectives (Global 2009) (CD-ROM), p.1081 - 1085, 2009/09
We have been developing an advanced reprocessing system for spent FBR fuels based on precipitation method using pyrrolidone derivatives, which consists of two precipitation steps; the first selective U precipitation step and the second U-Pu co-precipitation step. In the present study, precipitation behavior of Pu with pyrrolidone derivatives with high precipitation ability of N-(1,2-dimethyl)propyl-2-pyrrolidone (NDMProP), N-neopenthyl-2-pyrrolidone (NNpP) and N-cyclohexyl-2-pyrrolidone (NCP) has been examined with solutions of U-Pu mixture in order to evaluate their applicability to the second step. Since NNpP showed the highest precipitation ability for Pu(IV) and the best physical property as precipitate, NNpP would be the most appropriate precipitant for the U-Pu co-precipitation process. Precipitation of Np(IV, V, VI) with NNpP was also examined and it was found that Np(VI) could be quantitatively co-precipitated with U(VI) and Pu(IV).
Shibata, Atsuhiro; Oyama, Koichi; Yano, Kimihiko; Nomura, Kazunori; Koyama, Tomozo; Nakamura, Kazuhito; Kikuchi, Toshiaki*; Homma, Shunji*
Journal of Nuclear Science and Technology, 46(2), p.204 - 209, 2009/02
Times Cited Count:7 Percentile:45.28(Nuclear Science & Technology)A new reprocessing system with 2-stage crystallization process has been developed. In the first stage of the system, U and Pu are recovered from dissolver solution by U-Pu co-crystallization. Laboratory scale experiments were carried out with U and Pu mixed solution and irradiated fuel dissolver solution to obtain fundamental data on U-Pu co-crystallization process. Pu co-crystallized with U, but crystallization yields of Pu were lower than those of U. FPs were separated from U and Pu by co-crystallization, and decontamination factors of Cs and Eu to U in crystal were over 100.
Chikazawa, Takahiro*; Kikuchi, Toshiaki*; Shibata, Atsuhiro; Koyama, Tomozo; Homma, Shunji*
Journal of Nuclear Science and Technology, 45(6), p.582 - 587, 2008/06
Times Cited Count:18 Percentile:74.54(Nuclear Science & Technology)Batch crystallization of uranyl nitrate is carried out in order to obtain fundamental data required for the development of reprocessing involving crystallization. Particular attention is paid to the development of a method for predicting the concentrations of uranium and nitric acid in the mother liquor and the amount of uranyl nitrate crystals produced. Initial concentrations of uranyl nitrate and nitric acid are 500-600 g/dm and 4-6 mol/dm
and 4-6 mol/dm , respectively, corresponding to the condition of a dissolver solution of spent fuel. Steady-state mass balance equations including the correlation equation for the equilibrium solubility of uranium nitrate are applied to the prediction. The calculated concentrations of uranium and nitric acid are in close agreement with the experimental ones. The recovery of uranium is accurately predicted by the calculated concentrations, with an error of less than 5%.
, respectively, corresponding to the condition of a dissolver solution of spent fuel. Steady-state mass balance equations including the correlation equation for the equilibrium solubility of uranium nitrate are applied to the prediction. The calculated concentrations of uranium and nitric acid are in close agreement with the experimental ones. The recovery of uranium is accurately predicted by the calculated concentrations, with an error of less than 5%.