Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kimata, Tetsuya*; Kakitani, Kenta*; Yamamoto, Shunya*; Shimoyama, Iwao; Matsumura, Daiju; Iwase, Akihiro*; Mao, W.*; Kobayashi, Tomohiro*; Yamaki, Tetsuya*; Terai, Takayuki*
Physical Review Materials (Internet), 6(3), p.035801_1 - 035801_7, 2022/03
Times Cited Count:7 Percentile:71.37(Materials Science, Multidisciplinary) irradiation
irradiationOkazaki, Hiroyuki*; Kakitani, Kenta*; Kimata, Tetsuya*; Idesaki, Akira*; Koshikawa, Hiroshi*; Matsumura, Daiju; Yamamoto, Shunya*; Yamaki, Tetsuya*
Journal of Chemical Physics, 152(12), p.124708_1 - 124708_5, 2020/03
Times Cited Count:4 Percentile:25.92(Chemistry, Physical)Kakitani, Kenta*; Kimata, Tetsuya*; Yamaki, Tetsuya*; Yamamoto, Shunya*; Matsumura, Daiju; Taguchi, Tomitsugu*; Terai, Takayuki*
Radiation Physics and Chemistry, 153, p.152 - 155, 2018/12
Times Cited Count:3 Percentile:30.05(Chemistry, Physical)Kimata, Tetsuya*; Kato, Sho*; Yamaki, Tetsuya; Yamamoto, Shunya; Kobayashi, Tomohiro*; Terai, Takayuki*
Surface & Coatings Technology, 306(Part A), p.123 - 126, 2016/11
Times Cited Count:13 Percentile:52.49(Materials Science, Coatings & Films)Platinum (Pt) nanoparticle catalysts with oxygen reduction reaction (ORR) activity are required for practical applications of polymer electrolyte fuel cells. We prepared Pt nanoparticles on an Ar -irradiated glassy carbon (GC) surface by a radio-frequency magnetron sputtering method to investigate the influence of the ion-induced lattice defects in GC on the ORR activity of the deposited Pt nanoparticles. Interestingly, the Pt nanoparticles on the irradiated surface exhibited ca. 2.5 times higher specific activity than those on the non-irradiated one. X-ray photoelectron spectroscopy suggested the interfacial Pt-C interaction occurring between the irradiated GC and Pt nanoparticles, which should be a reason for improvement of the ORR activity.
-irradiated glassy carbon (GC) surface by a radio-frequency magnetron sputtering method to investigate the influence of the ion-induced lattice defects in GC on the ORR activity of the deposited Pt nanoparticles. Interestingly, the Pt nanoparticles on the irradiated surface exhibited ca. 2.5 times higher specific activity than those on the non-irradiated one. X-ray photoelectron spectroscopy suggested the interfacial Pt-C interaction occurring between the irradiated GC and Pt nanoparticles, which should be a reason for improvement of the ORR activity.
Kimata, Tetsuya*; Kato, Sho*; Yamaki, Tetsuya; Yamamoto, Shunya; Hakoda, Teruyuki; Kobayashi, Tomohiro*; Suzuki, Akihiro*; Terai, Takayuki*
no journal, ,
Platinum (Pt) nanoparticles prepared by Pt-ion implantation in a glassy carbon (GC) substrate exhibited high durability as oxygen reduction catalyst. This result would originate from chemical and electronic interaction between the Pt atoms at the particle surface and the lattice defects in the GC substrate introduced by the ion implantation. We prepared Pt nanoparticles by RF magnetron sputtering on the GC substrate modified by Ar-ion irradiation, and then investigated the influence of Ar-ion irradiation on their electronic structure. X-ray photoelectron spectroscopy demonstrated that the formation of the Pt-C bonding at the interface was promoted by the pre-deposition Ar-ion irradiation of the GC substrate.
Kimata, Tetsuya*; Kato, Sho*; Yamaki, Tetsuya; Yamamoto, Shunya; Hakoda, Teruyuki; Kobayashi, Tomohiro*; Suzuki, Akihiro*; Terai, Takayuki*
no journal, ,
Platinum (Pt) nanoparticles prepared by Pt-ion implantation in a glassy carbon (GC) substrate exhibited high durability as oxygen reduction catalyst. This result would originate from chemical and electronic interaction between the Pt atoms at the particle surface and the lattice defects in the GC substrate introduced by the ion implantation. We prepared Pt nanoparticles by RF magnetron sputtering on the Ar-ion-irradiated GC substrate, and then investigated the influence of Ar-ion irradiation on their supporting state of Pt nanoparticles. X-ray photoelectron spectroscopy demonstrated that the formation of the Pt-C bonding at the interface was promoted by the pre-deposition Ar-ion irradiation of the GC substrate.
Kimata, Tetsuya*; Kato, Sho*; Yamaki, Tetsuya; Yamamoto, Shunya; Hakoda, Teruyuki; Kobayashi, Tomohiro*; Suzuki, Akihiro*; Terai, Takayuki*
no journal, ,
Platinum (Pt) nanoparticles prepared by ion implantation in a glassy carbon (GC) substrate exhibited high activity and durability as oxygen reduction reaction (ORR) catalyst. This result would originate from chemical and electronic interaction between the Pt atoms at the particle surface and the lattice defects in the GC substrate introduced by the ion implantation. In this study, therefore, we sputtered Pt nanoparticles on the Ar-ion-irradiated GC substrate and then investigated their catalytic activity and electronic structure in terms of the effects of the irradiation defects in the substrate. The Ar-ion irradiation of the GC substrate was found to improve the ORR activity in an acid solution and to promote the formation of the Pt-C bonding at the interface.
Yamaki, Tetsuya; Kato, Sho*; Kimata, Tetsuya*; Yamamoto, Shunya; Hakoda, Teruyuki; Kobayashi, Tomohiro*; Suzuki, Akihiro*; Terai, Takayuki*
no journal, ,
We have a strong motivation to pursue the possibility of using ion beam technology for the creation of nano-structured catalytic materials and their electrochemical device applications. My talk includes (1) the preparation of noble-metal nanoparticles in a glassy carbon (GC) substrate by the metal-ion implantation method, (2) heavy-ion-induced lattice defects in the GC substrate and their effect on the oxygen reduction reaction activity of the deposited platinum nanoparticles, and (3) the formation of polymer nanostructures and their potential as a catalyst support.
Kimata, Tetsuya*; Kato, Sho*; Yamaki, Tetsuya; Yamamoto, Shunya; Hakoda, Teruyuki; Kobayashi, Tomohiro*; Terai, Takayuki*
no journal, ,
There have been intensive studies in the development of platinum (Pt) nanoparticle catalyst with high electrocatalytic activity and durability for fuel cell applications. We prepared the Pt nanoparticles on a glassy carbon (GC) substrate irradiated with 380 keV Ar , and then analyzed their electrocatalytic properties by a rotating disk electrode method. The Pt nanoparticles exhibited higher oxygen reduction reaction activity on the irradiated GC substrate than on the non-irradiated one. This suggests that the Ar-ion-induced modification of GC would improve the electrocatalytic properties of the deposited Pt nanoparticles.
, and then analyzed their electrocatalytic properties by a rotating disk electrode method. The Pt nanoparticles exhibited higher oxygen reduction reaction activity on the irradiated GC substrate than on the non-irradiated one. This suggests that the Ar-ion-induced modification of GC would improve the electrocatalytic properties of the deposited Pt nanoparticles.
Kimata, Tetsuya*; Kato, Sho*; Yamaki, Tetsuya; Yamamoto, Shunya; Hakoda, Teruyuki; Kobayashi, Tomohiro*; Terai, Takayuki*
no journal, ,
Platinum (Pt) nanoparticles with high oxygen reduction reaction activity have been required for applications to polymer electrolyte fuel cells. We prepared the Pt nanoparticles on an Ar-ion-irradiated glassy carbon (GC) substrate by a radio-frequency sputtering method to investigate the influence of the ion-induced lattice defects in GC on the deposited Pt nanoparticles by X-ray photoelectron spectroscopy (XPS). Detailed examination of both the Pt 4f and C 1s XPS spectra demonstrated that Pt-C bonding interaction occurred at the interface between the irradiated GC and Pt nanoparticles, confirming the significant effect of the Ar-ion irradiation on the electronic properties of Pt.
Kimata, Tetsuya*; Kato, Sho*; Yamaki, Tetsuya; Yamamoto, Shunya; Hakoda, Teruyuki; Kobayashi, Tomohiro*; Terai, Takayuki*
no journal, ,
Platinum (Pt) nanoparticles having high activities for an oxygen reduction reaction have been studied intensively for applications to polymer electrolyte fuel cells. We prepared the Pt nanoparticles on a glassy carbon (GC) substrate irradiated with 380 keV Ar at the fluence of 1.0
at the fluence of 1.0 10
10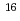 ions/cm
ions/cm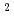 , and then analyzed their electrocatalytic properties by a rotating disk electrode method. The Pt nanoparticles on the Ar-ion-irradiated GC substrate exhibited ca. 2.5 times higher current density than those on the non-irradiated one. This striking result suggests that the Ar-ion-induced modification of the GC substrate would improve the electrocatalytic activities of the deposited Pt nanoparticles.
, and then analyzed their electrocatalytic properties by a rotating disk electrode method. The Pt nanoparticles on the Ar-ion-irradiated GC substrate exhibited ca. 2.5 times higher current density than those on the non-irradiated one. This striking result suggests that the Ar-ion-induced modification of the GC substrate would improve the electrocatalytic activities of the deposited Pt nanoparticles.
Kimata, Tetsuya*; Yamaki, Tetsuya; Yamamoto, Shunya; Hakoda, Teruyuki; Matsumura, Daiju; Shimoyama, Iwao; Iwase, Akihiro*; Fujimura, Yuki*; Kobayashi, Tomohiro*; Terai, Takayuki*
no journal, ,
Carbon-supported platinum (Pt) nanoparticles have been studied intensively for applications to oxygen reduction reaction (ORR) catalysts in polymer electrolyte fuel cells. The Pt nanoparticles on the Ar-ion-irradiated glassy carbon (GC) substrate were previously found to improve ORR activity. We analyzed here the local structure by XAFS measurements to clarify the mechanism of the Pt-C bonding at the Pt/GC interface, which could contribute to the observed high ORR activity. A decrease in the white-line intensity at Pt M and L
and L edges suggests the reduction of the vacancy in the Pt 5d orbital, thereby leading to the interfacial structure with suppressed oxygen adsorbability.
edges suggests the reduction of the vacancy in the Pt 5d orbital, thereby leading to the interfacial structure with suppressed oxygen adsorbability.
Kakitani, Kenta*; Kimata, Tetsuya*; Yamaki, Tetsuya; Yamamoto, Shunya; Terai, Takayuki*; Kobayashi, Tomohiro*
no journal, ,
Platinum (Pt) nanoparticles with high oxygen reduction reaction (ORR) activity have been required for applications to polymer electrolyte fuel cells. We prepared the Pt nanoparticles on a glassy carbon (GC) substrate irradiated with 380 keV Ar to tune the electronic structure of Pt nanoparticles and enhance the ORR activity. Rotating disk electrode measurements demonstrated that the Pt nanoparticles exhibited 2.5 times higher current density on the irradiated GC substrate than on the non-irradiated one. This improvement of the activity could be attributed to the formation of Pt-C bonding at the Pt/GC interface.
to tune the electronic structure of Pt nanoparticles and enhance the ORR activity. Rotating disk electrode measurements demonstrated that the Pt nanoparticles exhibited 2.5 times higher current density on the irradiated GC substrate than on the non-irradiated one. This improvement of the activity could be attributed to the formation of Pt-C bonding at the Pt/GC interface.
Kimata, Tetsuya*; Yamaki, Tetsuya; Yamamoto, Shunya; Matsumura, Daiju; Shimoyama, Iwao; Terai, Takayuki*; Iwase, Akihiro*; Fujimura, Yuki*; Kobayashi, Tomohiro*; Hakoda, Teruyuki
no journal, ,
Carbon-supported platinum (Pt) nanoparticles have been studied intensively for applications to oxygen reduction reaction (ORR) catalysts in polymer electrolyte fuel cells. The Pt nanoparticles on the Ar-ion-irradiated glassy carbon (GC) substrate were previously found to have improved ORR activity. We analyzed here the local structure by XAFS measurements to clarify the mechanism of the Pt-C bonding at the Pt/GC interface, which could contribute to the observed high ORR activity. EXAFS of Pt L edge demonstrated that the Pt-Pt bond length in the Pt nanoparticles was shorter on the Ar-ion-irradiated GC substrate than on the non-irradiated one. Therefore, the formation of Pt-C bonding would modify the structure of the Pt nanoparticles, thereby improving the ORR activity of the Pt nanoparticles.
edge demonstrated that the Pt-Pt bond length in the Pt nanoparticles was shorter on the Ar-ion-irradiated GC substrate than on the non-irradiated one. Therefore, the formation of Pt-C bonding would modify the structure of the Pt nanoparticles, thereby improving the ORR activity of the Pt nanoparticles.
 -irradiated carbon support
-irradiated carbon supportKakitani, Kenta*; Kimata, Tetsuya*; Yamaki, Tetsuya*; Yamamoto, Shunya*; Taguchi, Tomitsugu*; Shimoyama, Iwao; Matsumura, Daiju; Iwase, Akihiro*; Kobayashi, Tomohiro*; Terai, Takayuki*; et al.
no journal, ,
Pt nanoparticles (NPs) on the Ar -irradiated carbon support were found highly active for the oxygen reduction reaction (ORR). This suggests that irradiation defects of the support would affect Pt NPs, but the mechanism of the activity improvement has not been clear. We performed here transmission electron microscopy and X-ray absorption near edge structure (XANES) measurements to investigate the effect of the Pt NP-support interface in terms of the size and chemical states of the Pt NPs. The Pt NPs were prepared on the glassy carbon substrate by sputter deposition; their sizes were 5.1 and 3.8 nm on the 380 keV Ar
-irradiated carbon support were found highly active for the oxygen reduction reaction (ORR). This suggests that irradiation defects of the support would affect Pt NPs, but the mechanism of the activity improvement has not been clear. We performed here transmission electron microscopy and X-ray absorption near edge structure (XANES) measurements to investigate the effect of the Pt NP-support interface in terms of the size and chemical states of the Pt NPs. The Pt NPs were prepared on the glassy carbon substrate by sputter deposition; their sizes were 5.1 and 3.8 nm on the 380 keV Ar -irradiated and pristine substrates, respectively. In XANES spectra at Pt M
-irradiated and pristine substrates, respectively. In XANES spectra at Pt M and L
and L edges, the peak intensity was lower for the irradiated sample, indicating the suppression of Pt oxidation by the irradiation defects. Accordingly, fast kinetics originating from this interfacial chemical-state change, not an increase in the particle size, can explain the high ORR activity.
edges, the peak intensity was lower for the irradiated sample, indicating the suppression of Pt oxidation by the irradiation defects. Accordingly, fast kinetics originating from this interfacial chemical-state change, not an increase in the particle size, can explain the high ORR activity.
Kimata, Tetsuya*; Kakitani, Kenta*; Yamamoto, Shunya*; Taguchi, Tomitsugu*; Matsumura, Daiju; Shimoyama, Iwao; Iwase, Akihiro*; Kobayashi, Tomohiro*; Yamaki, Tetsuya*; Terai, Takayuki*
no journal, ,
Quite recently, the Pt nanoparticles on the Ar-ion-irradiated glassy carbon (GC) substrate have been found to exhibit high oxygen reduction reaction (ORR) activity due to electronic and structural effects through the irradiation lattice defects in the support. We performed X-ray absorption fine structure (XAFS) measurements to investigate the interface between GC and the Pt nanoparticles. The extended X-ray absorption fine structure at the Pt L edge demonstrated that the Pt-Pt bond length in the Pt nanoparticles was shorter on the Ar-ion-irradiated GC substrate than on the non-irradiated one. Therefore, the electronic interaction at the interface would modify the atomic structure of the supported Pt nanoparticles, thereby improving their ORR activity. This invited talk, called the JSAP young scientist presentation award speech, reviews what our strategy of the XAFS measurements should be for the mechanistic understanding of the GC/Pt-nanoparticle interface and enhanced ORR activity.
edge demonstrated that the Pt-Pt bond length in the Pt nanoparticles was shorter on the Ar-ion-irradiated GC substrate than on the non-irradiated one. Therefore, the electronic interaction at the interface would modify the atomic structure of the supported Pt nanoparticles, thereby improving their ORR activity. This invited talk, called the JSAP young scientist presentation award speech, reviews what our strategy of the XAFS measurements should be for the mechanistic understanding of the GC/Pt-nanoparticle interface and enhanced ORR activity.