Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
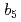
Hirano, Yu; Kimura, Shigenobu*; Tamada, Taro
Acta Crystallographica Section D, 71(7), p.1572 - 1581, 2015/07
Times Cited Count:3 Percentile:27.99(Biochemical Research Methods)Mammalian microsomal cytochrome 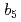 has multiple electron-transfer partners that function in various electron-transfer reactions. Four crystal structures of the solubilized haem-binding domain of cytochrome
has multiple electron-transfer partners that function in various electron-transfer reactions. Four crystal structures of the solubilized haem-binding domain of cytochrome 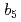 from porcine liver were determined at sub-angstrom resolution (0.76-0.95
from porcine liver were determined at sub-angstrom resolution (0.76-0.95  ) in two crystal forms for both the oxidized and reduced states. The high-resolution structures clearly displayed the electron density of H atoms in some amino-acid residues. Unrestrained refinement of bond lengths revealed that the protonation states of the haem propionate group may be involved in regulation of the haem redox properties. The haem Fe coordination geometry did not show significant differences between the oxidized and reduced structures. However, structural differences between the oxidized and reduced states were observed in the hydrogen-bond network around the axial ligand His68. The hydrogen-bond network could be involved in regulating the redox states of the haem group.
) in two crystal forms for both the oxidized and reduced states. The high-resolution structures clearly displayed the electron density of H atoms in some amino-acid residues. Unrestrained refinement of bond lengths revealed that the protonation states of the haem propionate group may be involved in regulation of the haem redox properties. The haem Fe coordination geometry did not show significant differences between the oxidized and reduced structures. However, structural differences between the oxidized and reduced states were observed in the hydrogen-bond network around the axial ligand His68. The hydrogen-bond network could be involved in regulating the redox states of the haem group.

 reductase by X-ray crystallography; New insights into regulation of efficient electron transfer
reductase by X-ray crystallography; New insights into regulation of efficient electron transferYamada, Mitsugu*; Tamada, Taro; Takeda, Kazuki*; Matsumoto, Fumiko*; Ono, Hiraku*; Kosugi, Masayuki*; Takaba, Kiyofumi*; Shoyama, Yoshinari*; Kimura, Shigenobu*; Kuroki, Ryota; et al.
Journal of Molecular Biology, 425(22), p.4295 - 4306, 2013/11
Times Cited Count:24 Percentile:52.94(Biochemistry & Molecular Biology)NADH-Cytochrome 
 reductase (b5R), a flavoprotein consisting of NADH and flavin adenine dinucleotide (FAD) binding domains, catalyzes electron transfer from the two-electron carrier NADH to the one-electron carrier cytochrome
reductase (b5R), a flavoprotein consisting of NADH and flavin adenine dinucleotide (FAD) binding domains, catalyzes electron transfer from the two-electron carrier NADH to the one-electron carrier cytochrome 
 (Cb5). The crystal structures of both the fully reduced form and the oxidized form of porcine liver b5R were determined. In the reduced b5R structure determined at 1.68
(Cb5). The crystal structures of both the fully reduced form and the oxidized form of porcine liver b5R were determined. In the reduced b5R structure determined at 1.68 resolution, the relative configuration of the two domains was slightly shifted in comparison with that of the oxidized form. This shift resulted in an increase in the solvent-accessible surface area of FAD and created a new hydrogen-bonding interaction between the N5 atom of the isoalloxazine ring of FAD and the hydroxyl oxygen atom of Thr66, which is considered to be a key residue in the release of a proton from the N5 atom. The isoalloxazine ring of FAD in the reduced form is flat as in the oxidized form and stacked together with the nicotinamide ring of NAD
resolution, the relative configuration of the two domains was slightly shifted in comparison with that of the oxidized form. This shift resulted in an increase in the solvent-accessible surface area of FAD and created a new hydrogen-bonding interaction between the N5 atom of the isoalloxazine ring of FAD and the hydroxyl oxygen atom of Thr66, which is considered to be a key residue in the release of a proton from the N5 atom. The isoalloxazine ring of FAD in the reduced form is flat as in the oxidized form and stacked together with the nicotinamide ring of NAD . Determination of the oxidized b5R structure, including the hydrogen atoms, determined at 0.78
. Determination of the oxidized b5R structure, including the hydrogen atoms, determined at 0.78 resolution revealed the details of a hydrogen-bonding network from the N5 atom of FAD to His49 via Thr66. Both of the reduced and oxidized b5R structures explain how backflow in this catalytic cycle is prevented and the transfer of electrons to one-electron acceptors such as Cb5 is accelerated. Furthermore, crystallographic analysis by the cryo-trapping method suggests that re-oxidation follows a two-step mechanism. These results provide structural insights into the catalytic cycle of b5R.
resolution revealed the details of a hydrogen-bonding network from the N5 atom of FAD to His49 via Thr66. Both of the reduced and oxidized b5R structures explain how backflow in this catalytic cycle is prevented and the transfer of electrons to one-electron acceptors such as Cb5 is accelerated. Furthermore, crystallographic analysis by the cryo-trapping method suggests that re-oxidation follows a two-step mechanism. These results provide structural insights into the catalytic cycle of b5R.
 reductase by X-ray crystallography
reductase by X-ray crystallographyTamada, Taro; Yamada, Mitsugu*; Takeda, Kazuki*; Matsumoto, Fumiko*; Kimura, Shigenobu*; Kuroki, Ryota; Miki, Kunio*
no journal, ,
NADH-cytochrome  reductase (b5R) is a flavoprotein consisting of NADH- and FAD- domains, and catalyzes the electron transfer from two electron carriers of NADH to one electron carrier of cytochrome
reductase (b5R) is a flavoprotein consisting of NADH- and FAD- domains, and catalyzes the electron transfer from two electron carriers of NADH to one electron carrier of cytochrome  (Cb5). The structure of the fully reduced form of porcine liver b5R was determined using a crystal grown under anaerobic condition. In the reduced b5R structure, which was determined at 1.68
(Cb5). The structure of the fully reduced form of porcine liver b5R was determined using a crystal grown under anaerobic condition. In the reduced b5R structure, which was determined at 1.68  resolution, the relative location of the two domains was slightly shifted in comparison with that of the oxidized form. This shift resulted in an increase in the solvent accessible surface area of FAD, and created a new hydrogen bonding interaction between the N5 atom of the isoalloxazine ring of FAD and the hydroxyl oxygen atom of Thr66, which is considered to be a key residue in the release of a proton from the N5 atom. The isoalloxazine ring of FAD in the reduced form is flat, which is similar to that in the oxidized form, and is stacked with the nicotinamide ring of NAD
resolution, the relative location of the two domains was slightly shifted in comparison with that of the oxidized form. This shift resulted in an increase in the solvent accessible surface area of FAD, and created a new hydrogen bonding interaction between the N5 atom of the isoalloxazine ring of FAD and the hydroxyl oxygen atom of Thr66, which is considered to be a key residue in the release of a proton from the N5 atom. The isoalloxazine ring of FAD in the reduced form is flat, which is similar to that in the oxidized form, and is stacked with the nicotinamide ring of NAD . Both of reduced and oxidized b5R structures could explain how prevents the backflow and accelerates the transfer of electrons to one-electron acceptors, such as Cb5, in the catalytic cycle. Furthermore, the crystallographic analysis by the cryo-trapping method, which controls the exposure time of the fully reduced crystals against the air, suggested that re-oxidation follows a two-step mechanism. These results provide structural insights into the catalytic cycle of b5R.
. Both of reduced and oxidized b5R structures could explain how prevents the backflow and accelerates the transfer of electrons to one-electron acceptors, such as Cb5, in the catalytic cycle. Furthermore, the crystallographic analysis by the cryo-trapping method, which controls the exposure time of the fully reduced crystals against the air, suggested that re-oxidation follows a two-step mechanism. These results provide structural insights into the catalytic cycle of b5R.
 from porcine liver
from porcine liverHirano, Yu; Kimura, Shigenobu*; Tamada, Taro
no journal, ,
Mammalian microsomal cytochrome  (b5) is a hemoprotein which anchored to the membrane of the endoplasmic reticulum and it is involved in various electron transfer reactions, such as lipid unsaturation, cholesterol synthesis and drug metabolism. The 1.5
(b5) is a hemoprotein which anchored to the membrane of the endoplasmic reticulum and it is involved in various electron transfer reactions, such as lipid unsaturation, cholesterol synthesis and drug metabolism. The 1.5  , structure of b5 from bovine liver reveals that acidic residues located in the vicinity of heme play a role in interaction with electron transfer partners. To further understand the electron transfer mechanism based on the precious structural information, we attempted the structure determination above 1
, structure of b5 from bovine liver reveals that acidic residues located in the vicinity of heme play a role in interaction with electron transfer partners. To further understand the electron transfer mechanism based on the precious structural information, we attempted the structure determination above 1  , resolution of both oxidized (OX) and reduced (RD) form of porcine liver b5. We obtained four types of b5 crystals, OX with/without calcium ion (Ca
, resolution of both oxidized (OX) and reduced (RD) form of porcine liver b5. We obtained four types of b5 crystals, OX with/without calcium ion (Ca ) and RD with/without Ca
) and RD with/without Ca , and then collected each dataset above 1
, and then collected each dataset above 1  , resolution, 0.85
, resolution, 0.85  , (OX + Ca
, (OX + Ca ), 0.93
), 0.93  ,(OX - Ca
,(OX - Ca ), 0.85
), 0.85  , (RD + Ca
, (RD + Ca ), and 0.95
), and 0.95  , (RD - Ca
, (RD - Ca ). In the both structures of OX and RD form with Ca
). In the both structures of OX and RD form with Ca , two glutamate located in the vicinity of heme participated in the recognition of Ca
, two glutamate located in the vicinity of heme participated in the recognition of Ca . From the comparison among four structures, we confirmed that the structural difference between with and without Ca
. From the comparison among four structures, we confirmed that the structural difference between with and without Ca (RMSD: 0.61-0.65
(RMSD: 0.61-0.65  ) is larger than that between OX and RD form (RMSD: 0.12-0.23
) is larger than that between OX and RD form (RMSD: 0.12-0.23  ).
).
 reductase at J-PARC
reductase at J-PARCHirano, Yu; Yamada, Mitsugu*; Kurihara, Kazuo; Kusaka, Katsuhiro*; Kimura, Shigenobu*; Miki, Kunio*; Tamada, Taro
no journal, ,
NADH-cytochrome  reductase (b5R), a flavoprotein consisting of NADH- and FAD-domains, catalyzes electron transfer from the two-electron carrier NADH to the one-electron carrier cytochrome
reductase (b5R), a flavoprotein consisting of NADH- and FAD-domains, catalyzes electron transfer from the two-electron carrier NADH to the one-electron carrier cytochrome  . The reaction catalyzed by b5R plays a role in fatty acid synthesis, cholesterol synthesis, and xenobiotic oxidation as a member of the electron transport chain on the endoplasmic reticulum. We have already determined the crystal structures of both the fully reduced and oxidized forms of porcine liver b5R by X-ray crystallography, but, its detail mechanism, especially hydride/proton transfers and exact states of semiquinone, still remains unknown. The hydrogen information obtained by neutron crystallography will be essential for the real understanding of catalytic cycle of the b5R. For neutron diffraction experiments, we prepared large crystals with the size of almost 2 mm
. The reaction catalyzed by b5R plays a role in fatty acid synthesis, cholesterol synthesis, and xenobiotic oxidation as a member of the electron transport chain on the endoplasmic reticulum. We have already determined the crystal structures of both the fully reduced and oxidized forms of porcine liver b5R by X-ray crystallography, but, its detail mechanism, especially hydride/proton transfers and exact states of semiquinone, still remains unknown. The hydrogen information obtained by neutron crystallography will be essential for the real understanding of catalytic cycle of the b5R. For neutron diffraction experiments, we prepared large crystals with the size of almost 2 mm . Large crystals were transferred to cryo-protectant solution by stepwise soaking method, and then were flash-frozen in a cold nitrogen gas stream. In preliminary neutron experiment, we confirmed some diffraction spots above 1.4
. Large crystals were transferred to cryo-protectant solution by stepwise soaking method, and then were flash-frozen in a cold nitrogen gas stream. In preliminary neutron experiment, we confirmed some diffraction spots above 1.4  , resolution from a crystal with the size of 1.8 mm
, resolution from a crystal with the size of 1.8 mm under 100 K at BL03 (iBIX), MLF, J-PARC after 14 hours exposure at 300 KW accelerator power.
under 100 K at BL03 (iBIX), MLF, J-PARC after 14 hours exposure at 300 KW accelerator power.
 reductase
reductaseHirano, Yu; Yamada, Mitsugu*; Kurihara, Kazuo; Shoyama, Yoshinari*; Kuroki, Ryota; Kusaka, Katsuhiro*; Kimura, Shigenobu*; Takeda, Kazuki*; Miki, Kunio*; Tamada, Taro
no journal, ,
NADH-cytochrome  reductase (b5R), a flavoprotein consisting of NADH- and FAD- domains, catalyzes electron transfer from the two-electron carrier NADH to the one-electron carrier cytochrome
reductase (b5R), a flavoprotein consisting of NADH- and FAD- domains, catalyzes electron transfer from the two-electron carrier NADH to the one-electron carrier cytochrome  . The reaction catalyzed by
. The reaction catalyzed by  plays a role in fatty acid synthesis, cholesterol synthesis, and xenobiotic oxidation as a member of the electron transport chain on the endoplasmic reticulum. We have already determined the crystal structures of both the fully reduced and oxidized forms of porcine liver b5R by X-ray crystallography, but, its detail mechanism, especially hydride/proton transfers and exact states of semiquinone, still remains unknown. The hydrogen information obtained by neutron crystallography will be essential for the real understanding of catalytic cycle of the
plays a role in fatty acid synthesis, cholesterol synthesis, and xenobiotic oxidation as a member of the electron transport chain on the endoplasmic reticulum. We have already determined the crystal structures of both the fully reduced and oxidized forms of porcine liver b5R by X-ray crystallography, but, its detail mechanism, especially hydride/proton transfers and exact states of semiquinone, still remains unknown. The hydrogen information obtained by neutron crystallography will be essential for the real understanding of catalytic cycle of the  . A large crystal with the size of almost 2 mm
. A large crystal with the size of almost 2 mm was transferred to cryo-protectant solution by stepwise soaking method, and then were flash-frozen in a cold nitrogen gas stream. Using this crystal, we collected neutron data to 1.4
was transferred to cryo-protectant solution by stepwise soaking method, and then were flash-frozen in a cold nitrogen gas stream. Using this crystal, we collected neutron data to 1.4 resolution at BL03 (iBIX), MLF, J-PARC, and then collected to 0.85
resolution at BL03 (iBIX), MLF, J-PARC, and then collected to 0.85 resolution at BL5A, PF, KEK. Crystallographic refinement using both neutron and X-ray data is in progress.
resolution at BL5A, PF, KEK. Crystallographic refinement using both neutron and X-ray data is in progress.
Yamada, Mitsugu; Tamada, Taro; Matsumoto, Fumiko; Takeda, Kazuki*; Kimura, Shigenobu*; Kuroki, Ryota; Miki, Kunio*
no journal, ,
no abstracts in English
 reductase
reductaseYamada, Mitsugu; Tamada, Taro; Matsumoto, Fumiko; Takeda, Kazuki*; Kimura, Shigenobu*; Kuroki, Ryota; Miki, Kunio*
no journal, ,
NADH-cytochrome  reductase (b5R; EC 1.6.2.2) is a flavoprotein and catalyses two-electron transfer from NADH to cytochrome
reductase (b5R; EC 1.6.2.2) is a flavoprotein and catalyses two-electron transfer from NADH to cytochrome  through FAD. Here, we present crystal structures of fully reduced and two re-oxidized forms of b5Rs determined. The crystal structure of fully reduced b5R showed that the relative location of two domains slightly shifted in comparison with that of oxidized form. This shift allowed to create a new hydrogen bonding interaction between N5 atom of isoalloxazine ring and hydroxyl oxygen atom of Thr66. The isoalloxazine ring of FAD in fully reduced form is flat and stacked with the nicotinamide ring of bound NAD
through FAD. Here, we present crystal structures of fully reduced and two re-oxidized forms of b5Rs determined. The crystal structure of fully reduced b5R showed that the relative location of two domains slightly shifted in comparison with that of oxidized form. This shift allowed to create a new hydrogen bonding interaction between N5 atom of isoalloxazine ring and hydroxyl oxygen atom of Thr66. The isoalloxazine ring of FAD in fully reduced form is flat and stacked with the nicotinamide ring of bound NAD . Moreover, two re-oxidized forms (form-1 and 2) were prepared using fully reduced crystals by exposure to the air. The electron densities for the nicotinamide ring of NAD
. Moreover, two re-oxidized forms (form-1 and 2) were prepared using fully reduced crystals by exposure to the air. The electron densities for the nicotinamide ring of NAD was ambiguous in form-1 and was completely disappeared in form-2. These results suggested that re-oxidization follows a two-step mechanism in which nicotinamide moiety is released firstly and then whole NAD
was ambiguous in form-1 and was completely disappeared in form-2. These results suggested that re-oxidization follows a two-step mechanism in which nicotinamide moiety is released firstly and then whole NAD is released.
is released.

 reductase aimed to neutron crystallography
reductase aimed to neutron crystallographyShoyama, Yoshinari; Tamada, Taro; Kuroki, Ryota; Kimura, Shigenobu*; Takeda, Kazuki*; Hayashi, Takuro*; Miki, Kunio*
no journal, ,
NADH-cytochrome 
 reductase (b5R) is a pyridine nucleotide-dependent flavin reductase which contains a FAD in a molecule. The b5R catalyzes the electron transfer from NADH to cytochrome b5. The b5R is related to fatty acid metabolism and reduction of cytochrome P450. In order to clarify detailed structure of b5R, we carried out purification of b5R from E coli cell according to the method previously reported. Since a large-size crystal is necessary for the neutron structure analysis. We addressed enlargement of crystal by macroseeding method and periodical addition of protein sample to the crystallization solution. We succeeded in a preparation of large-scale crystal with volume of 4 mm
reductase (b5R) is a pyridine nucleotide-dependent flavin reductase which contains a FAD in a molecule. The b5R catalyzes the electron transfer from NADH to cytochrome b5. The b5R is related to fatty acid metabolism and reduction of cytochrome P450. In order to clarify detailed structure of b5R, we carried out purification of b5R from E coli cell according to the method previously reported. Since a large-size crystal is necessary for the neutron structure analysis. We addressed enlargement of crystal by macroseeding method and periodical addition of protein sample to the crystallization solution. We succeeded in a preparation of large-scale crystal with volume of 4 mm , and we did preliminary neutron diffraction study using this crystal. Then we got 2
, and we did preliminary neutron diffraction study using this crystal. Then we got 2 resolution neutron diffraction data at BIX4.
resolution neutron diffraction data at BIX4.

Hirano, Yu; Kimura, Shigenobu*; Tamada, Taro
no journal, ,
Cytochrome  (Cyt
(Cyt  ) consisting of approximately 134 amino acid residues is a heme-binding protein and binds to the endoplasmic reticulum (ER) membrane. The N-terminal region of Cyt
) consisting of approximately 134 amino acid residues is a heme-binding protein and binds to the endoplasmic reticulum (ER) membrane. The N-terminal region of Cyt  is the heme-binding domain in the cytoplasmic side of ER membrane and the C-terminal region is the membrane-binding domain. We performed X-ray crystal structure analyses of the N-terminal 94 residues of the Cyt
is the heme-binding domain in the cytoplasmic side of ER membrane and the C-terminal region is the membrane-binding domain. We performed X-ray crystal structure analyses of the N-terminal 94 residues of the Cyt  from porcine liver in both oxidized and reduced states. Diffraction experiments were carried out at the BL17A beamline of PF. We have obtained the data sets at 0.85
from porcine liver in both oxidized and reduced states. Diffraction experiments were carried out at the BL17A beamline of PF. We have obtained the data sets at 0.85  , resolution (+Ca) and 0.93
, resolution (+Ca) and 0.93  , resolution (-Ca) for the oxidized form and at 0.85
, resolution (-Ca) for the oxidized form and at 0.85  , resolution (+Ca) and 0.98
, resolution (+Ca) and 0.98  , resolution (-Ca) for the reduced form. Structure refinement revealed that the side chains of two glutamic acid residues near the heme group were involved in the coordination of a Ca ion in the crystal structure with Ca ions.
, resolution (-Ca) for the reduced form. Structure refinement revealed that the side chains of two glutamic acid residues near the heme group were involved in the coordination of a Ca ion in the crystal structure with Ca ions.
Shoyama, Yoshinari; Tamada, Taro; Kuroki, Ryota; Kimura, Shigenobu*; Takeda, Kazuki*; Hayashi, Takuro*; Miki, Kunio*
no journal, ,
NADH-cytochrome b5 reductase (b5R) is a pyridine nucleotide-dependent flavin reductase which contains a FAD in a molecule. The b5R catalyzes the electron transfer from NADH to cytochrome b5. The b5R is related to fatty acid metabolism and reduction of cytochrome P450. In order to clarify detailed structure of b5R, we carried out purification of b5R from E coli cell according to the method previously reported. Since a large-size crystal is necessary for the neutron structure analysis, we addressed enlargement of crystal by macroseeding method and periodical addition of protein sample to the crystallization solution. We succeeded in a preparation of large-scale crystal with a size of 1.0 0.5
0.5 0.3 mm, which is suitable for preliminary neutron diffraction study.
0.3 mm, which is suitable for preliminary neutron diffraction study.
Hirano, Yu; Kimura, Shigenobu*; Tamada, Taro
no journal, ,
Mammalian microsomal cytochrome b5 has multiple electron-transfer partners that function in various electron-transfer reactions. X-ray diffraction data of the solubilized haem-binding domain of cytochrome b5 from porcine liver were collected at PF BL5A and BL17A beamlines. We obtained four data sets at sub-angstrom resolution in two crystal forms for both the oxidized and reduced states. The high-resolution structures clearly displayed the electron density of H atoms in some amino-acid residues. Unrestrained refinement of bond lengths revealed that the protonation states of the haem propionate group may be involved in regulation of the haem redox properties. Although the haem Fe coordination geometry did not show significant differences between the oxidized and reduced structures, structural differences were observed in the hydrogen-bond network around the axial ligand His68. The hydrogen-bond network could be involved in regulating the redox states of the haem group.
 reductase
reductaseYamada, Mitsugu; Tamada, Taro; Matsumoto, Fumiko; Takeda, Kazuki*; Kimura, Shigenobu*; Kuroki, Ryota; Miki, Kunio*
no journal, ,
no abstracts in English