Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kimura, Taiki*; Kaneko, Masashi; Watanabe, Masayuki; Miyashita, Sunao*; Nakashima, Satoru*
Dalton Transactions (Internet), 47(42), p.14924 - 14931, 2018/11
Times Cited Count:9 Percentile:43.89(Chemistry, Inorganic & Nuclear)We demonstrated density functional calculations of Eu(III) and Am(III) complexes with pnictogen-donor (X) ligands, CH )
) X-CH
X-CH -CH
-CH -X(CH
-X(CH )
) (X = N, P, As and Sb). We investigated the optimized structures of the cmoplexes and the Gibbs energy differences in the complex formation reactions. Those results indicated that the N- and P-donor ligands have Am(III) ion selectivity over Eu(III) ion, especially, the P-donor ligand showed the highest selectivity. The tendency of the Am(III)/Eu(III) selectivity by the pnictogen-dono ligands was found to be comparable to that of soft acid classification in hard and soft acids and bases rule. Mulliken's spin population analysis indicated that the bonding property between the metal ion and the pnictogen atoms correlated with the Am(III)/Eu(III) selectivity. In particular, the participation of f-orbital electrons of the metal ion in the covalency was indicated to have an important role for the selectivity.
(X = N, P, As and Sb). We investigated the optimized structures of the cmoplexes and the Gibbs energy differences in the complex formation reactions. Those results indicated that the N- and P-donor ligands have Am(III) ion selectivity over Eu(III) ion, especially, the P-donor ligand showed the highest selectivity. The tendency of the Am(III)/Eu(III) selectivity by the pnictogen-dono ligands was found to be comparable to that of soft acid classification in hard and soft acids and bases rule. Mulliken's spin population analysis indicated that the bonding property between the metal ion and the pnictogen atoms correlated with the Am(III)/Eu(III) selectivity. In particular, the participation of f-orbital electrons of the metal ion in the covalency was indicated to have an important role for the selectivity.
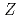 = 100 with a multireflection time-of-flight mass spectrograph
= 100 with a multireflection time-of-flight mass spectrographIto, Yuta*; Schury, P.*; Wada, Michiharu*; Arai, Fumiya*; Haba, Hiromitsu*; Hirayama, Yoshikazu*; Ishizawa, Satoshi*; Kaji, Daiya*; Kimura, Sota*; Koura, Hiroyuki; et al.
Physical Review Letters, 120(15), p.152501_1 - 152501_6, 2018/04
Times Cited Count:66 Percentile:92.72(Physics, Multidisciplinary)Masses of 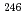 Es,
Es, 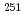 Fm and the transfermium nuclei
Fm and the transfermium nuclei 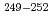 Md, and
Md, and 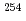 No, produced by hot- and cold-fusion reactions, in the vicinity of the deformed
No, produced by hot- and cold-fusion reactions, in the vicinity of the deformed 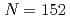 neutron shell closure, have been directly measured using a multi-reflection time-of-flight mass spectrograph. The masses of
neutron shell closure, have been directly measured using a multi-reflection time-of-flight mass spectrograph. The masses of 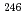 Es and
Es and 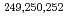 Md were measured for the first time. Using the masses of
Md were measured for the first time. Using the masses of 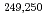 Md as anchor points for
Md as anchor points for  decay chains, the masses of heavier nuclei, up to
decay chains, the masses of heavier nuclei, up to  Bh and
Bh and 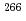 Mt, were determined. These new masses were compared with theoretical global mass models and demonstrated to be in good agreement with macroscopic-microscopic models in this region. The empirical shell gap parameter
Mt, were determined. These new masses were compared with theoretical global mass models and demonstrated to be in good agreement with macroscopic-microscopic models in this region. The empirical shell gap parameter 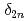 derived from three isotopic masses was updated with the new masses and corroborate the existence of the deformed
derived from three isotopic masses was updated with the new masses and corroborate the existence of the deformed 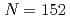 neutron shell closure for Md and Lr.
neutron shell closure for Md and Lr.
Schury, P.*; Wada, Michiharu*; Ito, Yuta*; Kaji, Daiya*; Haba, Hiromitsu*; Hirayama, Yoshikazu*; Kimura, Sota*; Koura, Hiroyuki; MacCormick, M.*; Miyatake, Hiroari*; et al.
Nuclear Instruments and Methods in Physics Research B, 407, p.160 - 165, 2017/06
Times Cited Count:16 Percentile:79.30(Instruments & Instrumentation)Various isotopes of Ac, Ra, Fr, and Rn were produced by fusion-evaporation reactions using a  Ca beam. The energetic ions were stopped in and extracted from a helium gas cell. The extracted ions were identified using a multi-reflection time-of-fight mass spectrograph. In all cases, it was observed that the predominant charge state for the extracted ions, including the alkali Fr, was 2+.
Ca beam. The energetic ions were stopped in and extracted from a helium gas cell. The extracted ions were identified using a multi-reflection time-of-fight mass spectrograph. In all cases, it was observed that the predominant charge state for the extracted ions, including the alkali Fr, was 2+.
Schury, P.*; Wada, Michiharu*; Ito, Yuta*; Kaji, Daiya*; Arai, Fumiya*; MacCormick, M.*; Murray, I.*; Haba, Hiromitsu*; Jeong, S.*; Kimura, Sota*; et al.
Physical Review C, 95(1), p.011305_1 - 011305_6, 2017/01
Times Cited Count:48 Percentile:94.99(Physics, Nuclear)Using a multireflection time-of-flight mass spectrograph located after a gas cell coupled with the gas-filled recoil ion separator GARIS-II, the masses of several  -decaying heavy nuclei were directly and precisely measured. The nuclei were produced via fusion-evaporation reactions and separated from projectilelike and targetlike particles using GARIS-II before being stopped in a helium-filled gas cell. Time-of-flight spectra for three isobar chains,
-decaying heavy nuclei were directly and precisely measured. The nuclei were produced via fusion-evaporation reactions and separated from projectilelike and targetlike particles using GARIS-II before being stopped in a helium-filled gas cell. Time-of-flight spectra for three isobar chains, 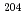 Fr-
Fr-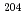 Rn-
Rn-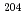 At-
At-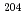 Po,
Po, 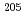 Fr-
Fr- 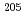 Rn-
Rn-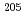 At-
At-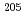 Po-
Po-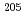 Bi, and
Bi, and  Fr-
Fr- Rn-
Rn- At, were observed. Precision atomic mass values were determined for
At, were observed. Precision atomic mass values were determined for 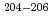 Fr,
Fr, 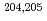 Rn, and
Rn, and 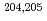 At. Identifications of
At. Identifications of 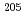 Bi,
Bi, 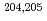 Po,
Po,  Rn, and
Rn, and  At were made with N
At were made with N 10 detected ions, representing the next step toward use of mass spectrometry to identify exceedingly low-yield species such as superheavy element ions.
10 detected ions, representing the next step toward use of mass spectrometry to identify exceedingly low-yield species such as superheavy element ions.
Iwamoto, Taiki*; Zhang, Yuan*; Kuroda, Masatoshi*; Hirota, Noriaki; Kobayashi, Yuji*; Kimura, Yuta*
no journal, ,
Nowadays, researches on quantitative evaluation of plastic strain and residual stress of stainless steel with various Electron Backscatter Diffraction (abbreviated to EBSD) parameters have been actively conducted. Generally, it is considered that residual stress accumulated near the grain boundary. It might be caused by dislocations and resulted in the increment of the average value of local misorientations. In this study, we focused on EBSD parameters and investigated the applicability of these parameters as an evaluation metric for the depth direction of residual stress in austenitic stainless steel subjected to laser peening.
Kaneko, Masashi; Kimura, Taiki; Watanabe, Masayuki; Miyashita, Sunao*; Nakashima, Satoru*
no journal, ,
We aim to developt the prediction code for separation performance of minor-actinides from lanthanides. In this study, we constructed a chemical bonding database of Am and Eu with Group 15 and 16 element donor ligands. As the result of energy analysis using density functional theory calculations, the trend of separation behavior of Am from Eu with group 15 and 16 donor ligands correlated with the trend of soft acid classification of hard and soft acids and bases principle. As the result of bonding analysis, the donor ligands which strongly bond to Am were indicated to possess the high selectivity to Am.