Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Kinoshita, Ryoma; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
Analytical Sciences, 39(9), p.1575 - 1583, 2023/09
Times Cited Count:0 Percentile:0(Chemistry, Analytical)Extraction of Rh from HCl can be performed by NTAamide(C6) (hexahexyl-nitrilotriacetamide) and other related compounds into n-dodecane. We use ion-pair extraction of anionic species of Rh-chloride and protonated extractant. Rh behave as anion in hydrochloric acid and the tertiary nitrogen atom in extractant may be protonated to produce the quaternary amine in acidic condition. From the present work, the maximum distribution ratio of Rh(III) is 16. The D(Rh) values are changeable during preparation of the aqueous solutions because different Rh-Cl-H O complexes are formed in HCl media and show the slow exchange rate between Cl and H
O complexes are formed in HCl media and show the slow exchange rate between Cl and H O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl
O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl (H
(H O)
O) and RhCl
and RhCl (H
(H O)
O)
 exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Kinoshita, Ryoma*; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
no journal, ,
We obtained relatively high D(Rh) of approximate 1 by iminodioctamide (IDOA) having tertially amino N atom from concentrated HCl solution. The reason is that IDOA is protonated, behaves as cationic extractant, and extracts anionic RhCl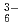 through ion-pair extraction. Up to now, there is less information on Rh(III) extraction, we investigate the behavior of Rh extraction and discuss with theoretical studies.
through ion-pair extraction. Up to now, there is less information on Rh(III) extraction, we investigate the behavior of Rh extraction and discuss with theoretical studies.
Kinoshita, Ryoma; Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
no journal, ,
Many metal ions are stable in hydrochloric acid solutions as anionic species; diamidic-extractants containing tertiary amino nitrogen atom, such as NTAamide (hexaalkyl-nitrilotriacetamide), are normally protonated in acidic solutions and become cationic extractants. In this study, the ion-pair extraction reactions of metal chloride anions with cationic NTAamide(C6) extractant were investigated. In addition, we attempted to predict the distribution ratio by combining the stability constants of metal chloride complexes and DFT calculations, and compared the results with experimental values.