Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Iwamoto, Osamu; Iwamoto, Nobuyuki; Kunieda, Satoshi; Minato, Futoshi; Nakayama, Shinsuke; Abe, Yutaka*; Tsubakihara, Kosuke*; Okumura, Shin*; Ishizuka, Chikako*; Yoshida, Tadashi*; et al.
Journal of Nuclear Science and Technology, 60(1), p.1 - 60, 2023/01
Times Cited Count:213 Percentile:99.99(Nuclear Science & Technology)Li, B.*; Kawakita, Yukinobu; Kawamura, Seiko; Sugahara, Takeshi*; Wang, H.*; Wang, J.*; Chen, Y.*; Kawaguchi, Saori*; Kawaguchi, Shogo*; Ohara, Koji*; et al.
Nature, 567(7749), p.506 - 510, 2019/03
Times Cited Count:327 Percentile:99.52(Multidisciplinary Sciences)Refrigeration is of vital importance for modern society for example, for food storage and air conditioning- and 25 to 30% of the world's electricity is consumed for refrigeration. Current refrigeration technology mostly involves the conventional vapour compression cycle, but the materials used in this technology are of growing environmental concern because of their large global warming potential. As a promising alternative, refrigeration technologies based on solid-state caloric effects have been attracting attention in recent decades. However, their application is restricted by the limited performance of current caloric materials, owing to small isothermal entropy changes and large driving magnetic fields. Here we report colossal barocaloric effects (CBCEs) (barocaloric effects are cooling effects of pressure-induced phase transitions) in a class of disordered solids called plastic crystals. The obtained entropy changes in a representative plastic crystal, neopentylglycol, are about 389 joules per kilogram per kelvin near room temperature. Pressure-dependent neutron scattering measurements reveal that CBCEs in plastic crystals can be attributed to the combination of extensive molecular orientational disorder, giant compressibility and highly anharmonic lattice dynamics of these materials. Our study establishes the microscopic mechanism of CBCEs in plastic crystals and paves the way to next-generation solid-state refrigeration technologies.
Li, B.; Wang, H.*; Kawakita, Yukinobu; Zhang, Q.*; Feygenson, M.*; Yu, H. L.*; Wu, D.*; Ohara, Koji*; Kikuchi, Tatsuya*; Shibata, Kaoru; et al.
Nature Materials, 17(3), p.226 - 230, 2018/03
Times Cited Count:161 Percentile:97.18(Chemistry, Physical)
Li, B.; Luo, X. H.*; Wang, H.*; Ren, W. J.*; Yano, S.*; Wang, C.-W.*; Gardner, J. S.*; Liss, K.-D.*; Miao, P.*; Lee, S.-H.*; et al.
Physical Review B, 93(22), p.224405_1 - 224405_6, 2016/06
Times Cited Count:56 Percentile:87.40(Materials Science, Multidisciplinary)Oe, Kazuhiro*; Attallah, M. F.*; Asai, Masato; Goto, Naoya*; Gupta, N. S.*; Haba, Hiromitsu*; Huang, M.*; Kanaya, Jumpei*; Kaneya, Yusuke*; Kasamatsu, Yoshitaka*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1317 - 1320, 2015/02
Times Cited Count:10 Percentile:60.46(Chemistry, Analytical)A new technique for continuous dissolution of nuclear reaction products transported by a gas-jet system was developed for superheavy element (SHE) chemistry. In this technique, a hydrophobic membrane is utilized to separate an aqueous phase from the gas phase. With this technique, the dissolution efficiencies of short-lived radionuclides of 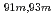 Mo and
Mo and 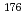 W were measured. Yields of more than 80% were observed for short-lived radionuclides at aqueous-phase flow rates of 0.1-0.4 mL/s. The gas flow-rate had no influence on the dissolution efficiency within the studied flow range of 1.0-2.0 L/min. These results show that this technique is applicable for on-line chemical studies of SHEs in the liquid phase.
W were measured. Yields of more than 80% were observed for short-lived radionuclides at aqueous-phase flow rates of 0.1-0.4 mL/s. The gas flow-rate had no influence on the dissolution efficiency within the studied flow range of 1.0-2.0 L/min. These results show that this technique is applicable for on-line chemical studies of SHEs in the liquid phase.
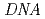 at the MLF, J-PARC
at the MLF, J-PARCTakahashi, Nobuaki; Shibata, Kaoru; Kawakita, Yukinobu; Nakajima, Kenji; Inamura, Yasuhiro; Nakatani, Takeshi; Nakagawa, Hiroshi; Fujiwara, Satoru; Sato, Taku*; Tsukushi, Itaru*; et al.
Journal of the Physical Society of Japan, 80(Suppl.B), p.SB007_1 - SB007_4, 2011/12
Times Cited Count:7 Percentile:45.98(Physics, Multidisciplinary)Shibata, Taiju; Sumita, Junya; Makita, Taiyo*; Takagi, Takashi*; Kunimoto, Eiji*; Sawa, Kazuhiro; Kim, W. J.*; Jung, C. H.*; Park, J. Y.*
Nihon Kikai Gakkai Rombunshu, A, 76(764), p.383 - 385, 2010/04
no abstracts in English
 thin films
thin filmsLee, H.-S.*; Okada, Hiroshi*; Wakahara, Akihiro*; Yoshida, Akira*; Oshima, Takeshi; Ito, Hisayoshi; Kawakita, Shiro*; Imaizumi, Mitsuru*; Matsuda, Sumio*
Physica Status Solidi (A), 199(3), p.471 - 474, 2003/10
Times Cited Count:3 Percentile:20.75(Materials Science, Multidisciplinary)no abstracts in English
 thin films
thin filmsLee, H.-S.*; Okada, Hiroshi*; Wakahara, Akihiro*; Oshima, Takeshi; Ito, Hisayoshi; Kawakita, Shiro*; Imaizumi, Mitsuru*; Matsuda, Sumio*; Yoshida, Akira*
Journal of Physics and Chemistry of Solids, 64(9-10), p.1887 - 1890, 2003/09
Times Cited Count:13 Percentile:55.19(Chemistry, Multidisciplinary)no abstracts in English
Shibamoto, Yasuteru; Kukita, Yutaka*; Nakamura, Hideo; Park, H. S.*; Anoda, Yoshinari
Proceedings of 8th International Conference on Nuclear Engineering (ICONE-8) (CD-ROM), p.12 - 0, 2000/00
no abstracts in English
Chen, C.*; Nishiyama, Takashi*; Kusuda, Hiromu*; Kita, H.*; Sato, Toshinori
International Journal of Rock Mechanics and Mining Sciences, 36(4), p.535 - 541, 1999/06
Times Cited Count:37 Percentile:87.73(Engineering, Geological)None
King, C.-Y.; Azuma, S.; Igarashi, G.; Saito, Hiroshi; Wakita, H.
Journal of Geophysical Research; Solid Earth, 104(B6), p.13073 - 13082, 1999/00
Times Cited Count:108 Percentile:86.31(Geochemistry & Geophysics)None
H.Lee*; H.Seong*; G.Park*; Kumamaru, Hiroshige; Kukita, Yutaka
Proc. of ASME JSME 4th Int. Conf. on Nuclear Engineering 1996 (ICONE-4), 3, p.41 - 50, 1996/00
JSME 4th Int. Conf. on Nuclear Engineering 1996 (ICONE-4), 3, p.41 - 50, 1996/00
no abstracts in English
Stumpf, H.*; F.Motley*; R.Schultz*; J.Chapman*; Kukita, Yutaka
Natural Circulation, p.103 - 109, 1987/00
no abstracts in English
P.R.Schulz*; J.C.Chapman*; Kukita, Yutaka; F.E.Motley*; Stumpf, H.*; Y.S.Chen*; Tasaka, Kanji
Natural Circulation, p.59 - 70, 1987/00
no abstracts in English
Shibata, Kaoru; Takahashi, Nobuaki; Nakajima, Kenji; Kambara, Wataru; Kawakita, Yukinobu*; Sato, Taku*; Tsukushi, Itaru*; Nakagawa, Hiroshi; Fujiwara, Satoru; Mezei, F.*; et al.
no journal, ,
no abstracts in English
Shibata, Kaoru*; Takahashi, Nobuaki; Kawakita, Yukinobu; Kamazawa, Kazuya*; Yamada, Takeshi*; Ueno, Hiroki; Shimakura, Hironori; Nakajima, Kenji; Kambara, Wataru; Inamura, Yasuhiro; et al.
no journal, ,
no abstracts in English
Takahashi, Nobuaki; Shibata, Kaoru*; Yamada, Takeshi*; Kamazawa, Kazuya*; Kawakita, Yukinobu; Nakajima, Kenji; Kambara, Wataru; Inamura, Yasuhiro; Nakatani, Takeshi; Aizawa, Kazuya; et al.
no journal, ,
A Si crystal analyzer near backscattering spectrometer DNA which covers the area of the micro-eV energy range in the Q-E space, where it is expected to explore sciences on atomic, molecular and spin dynamics in the nanosecond time range has been installed at BL02 of the Materials and Life Science Experimental Facility (MLF) of the Japan Proton Accelerator Research Complex (J-PARC) until at the end of February 2012. Then, various on-beam commissioning has been done for about a year with handling continuing user program. In this report, we will introduce specifications, current status of the instrument.
Toyoshima, Atsushi; Asai, Masato; Attallah, M. F.*; Goto, Naoya*; Gupta, N. S.*; Haba, Hiromitsu*; Huang, M.*; Kanaya, Jumpei*; Kaneya, Yusuke; Kasamatsu, Yoshitaka*; et al.
no journal, ,
Towards electrolytic reduction of Sg, batch-wise electrolytic reduction of carrier-free 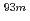 Mo and
Mo and 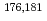 W radiotracers was studied using a flow electrolytic column (FEC). The electrolyzed samples from a FEC were chemically analyzed by solvent extraction with TOA and HDEHP to separate and identify reduced species from the stable Mo(VI) and W(VI) ones based on their different extraction behavior.
W radiotracers was studied using a flow electrolytic column (FEC). The electrolyzed samples from a FEC were chemically analyzed by solvent extraction with TOA and HDEHP to separate and identify reduced species from the stable Mo(VI) and W(VI) ones based on their different extraction behavior. 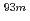 Mo and
Mo and 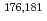 W were applied as radiotracers. We also performed cyclic voltammetry and UV/Vis absorption spectrometry of macro amounts of Mo and W in acidic solutions to obtain information on redox reactions of these elements under given conditions. In the conference, the present status of the preparatory reduction experiments with Mo and W will be presented.
W were applied as radiotracers. We also performed cyclic voltammetry and UV/Vis absorption spectrometry of macro amounts of Mo and W in acidic solutions to obtain information on redox reactions of these elements under given conditions. In the conference, the present status of the preparatory reduction experiments with Mo and W will be presented.
Toyoshima, Atsushi; Oe, Kazuhiro*; Asai, Masato; Attallah, M. F.*; Goto, Naoya*; Gupta, N. S.*; Haba, Hiromitsu*; Kaneko, Masashi*; Kaneya, Yusuke; Kasamatsu, Yoshitaka*; et al.
no journal, ,
Due to short half-lives less than 10 s and extremely low production rates, transactinide elements heavier than seaborgium (Sg) are produced on an atom per hour scale. Therefore, a continuous rapid chemistry assembly is required to study aqueous-phase chemistry of these heaviest elements. In the present study, we started developments of a continuous chemistry assembly. Our first attempt was made in on-line experiments with Mo and W, lighter homologs of Sg, to optimize a chemistry assembly consisting of a newly developed membrane degasser as an interface between gas-jet and aqueous phase, a flow electrolytic column apparatus utilized to control oxidation states of Mo and W ions, and the continuous liquid-liquid extraction apparatus of SISAK for separation. In the conference, present status of the developments will be presented.