Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Tc separation/concentration technology from
Tc separation/concentration technology from  Mo by (n,
Mo by (n,  ) method, 2
) method, 2Fujita, Yoshitaka; Hu, X.*; Yang, Y.*; Kitagawa, Taiga*; Fujihara, Yasuyuki*; Yoshinaga, Hisao*; Hori, Junichi*; Do, T. M. D.*; Suzuki, Tatsuya*; Suematsu, Hisayuki*; et al.
KURNS Progress Report 2023, P. 122, 2024/07
no abstracts in English
Tang, J.*; Seo, O.*; Rivera Rocabado, D. S.*; Koitaya, Takanori*; Yamamoto, Susumu*; Namba, Yusuke*; Song, C.*; Kim, J.*; Yoshigoe, Akitaka; Koyama, Michihisa*; et al.
Applied Surface Science, 587, p.152797_1 - 152797_8, 2022/06
Times Cited Count:11 Percentile:69.49(Chemistry, Physical)The hydrogen absorption and diffusion mechanisms on cube-shaped Pd nanoparticles (NPs) which are important hydrogen-storage materials were studied using X-ray photoelectron spectroscopy and DFT calculations. In the surface region, hydrogen absorption showed almost similar behavior regardless of the NPs size. It was found that the octahedral sites are more favorable than the tetrahedral sites for hydrogen occupation. We also clarified that the hydrogen atoms absorbing on the smaller-sized Pd NPs diffuse to the subsurface more actively because of the weakened Pd-H bond by the surface disordering, which plays an important role in hydrogen adsorption at a low H pressure.
pressure.
 dispersive X-ray absorption fine structure spectroscopy
dispersive X-ray absorption fine structure spectroscopySong, C.*; Seo, O.*; Matsumura, Daiju; Hiroi, Satoshi*; Cui, Y.-T.*; Kim, J.*; Chen, Y.*; Tayal, A.*; Kusada, Kohei*; Kobayashi, Hirokazu*; et al.
RSC Advances (Internet), 10(34), p.19751 - 19758, 2020/05
Times Cited Count:0 Percentile:0.00(Chemistry, Multidisciplinary) Rh
Rh )
) Al
Al (x = 0,0.1)
(x = 0,0.1)Tanida, Hiroshi*; Kitagawa, Kentaro*; Tateiwa, Naoyuki; Sera, Masafumi*; Nishioka, Takashi*
Physical Review B, 96(23), p.235131_1 - 235131_7, 2017/12
Times Cited Count:1 Percentile:5.34(Materials Science, Multidisciplinary)We have done high pressure experiments on Ce(Ru Rh
Rh )
) Al
Al (x = 0 and 0.1) to study pressure-induced phase transitions from the antiferromagnetic to paramagnetic state at
(x = 0 and 0.1) to study pressure-induced phase transitions from the antiferromagnetic to paramagnetic state at 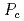 . Experimental data of the electrical resistivity suggest that the
. Experimental data of the electrical resistivity suggest that the 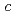 -
- hybridization gap could be not necessary to form the unusual AFM order. The pressure effects on the magnetic susceptibility is also studied. We discuss a difference in the pressure response of the magnetic susceptibility based the electronic state between x = 0 and 0.1.
hybridization gap could be not necessary to form the unusual AFM order. The pressure effects on the magnetic susceptibility is also studied. We discuss a difference in the pressure response of the magnetic susceptibility based the electronic state between x = 0 and 0.1.
Kofu, Maiko; Hashimoto, Naoki*; Akiba, Hiroshi*; Kobayashi, Hirokazu*; Kitagawa, Hiroshi*; Iida, Kazuki*; Nakamura, Mitsutaka; Yamamuro, Osamu*
Physical Review B, 96(5), p.054304_1 - 054304_7, 2017/08
Times Cited Count:19 Percentile:61.36(Materials Science, Multidisciplinary)The vibrational states of hydrogen atoms in bulk and nanocrystalline palladium were examined in a wide energy region 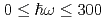 meV using neutron spectroscopy. In bulk PdH
meV using neutron spectroscopy. In bulk PdH , the vibrational excitations of H atoms were roughly reproduced by the quantum harmonic oscillator (QHO) model. In PdH
, the vibrational excitations of H atoms were roughly reproduced by the quantum harmonic oscillator (QHO) model. In PdH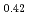 nanocrystals with a diameter of 8 nm, however, additional vibrational excitations were found at energies above 80 meV. The energies and intensities of the additional states were not explained by QHO but reasonably described as vibrations in a highly anharmonic trumpet-like potential. The additional excitations are attributed to the vibrations of H atoms at tetrahedral sites in the subsurface region stabilized by surface effects. This is an experimental work which clearly detects hydrogen vibration
nanocrystals with a diameter of 8 nm, however, additional vibrational excitations were found at energies above 80 meV. The energies and intensities of the additional states were not explained by QHO but reasonably described as vibrations in a highly anharmonic trumpet-like potential. The additional excitations are attributed to the vibrations of H atoms at tetrahedral sites in the subsurface region stabilized by surface effects. This is an experimental work which clearly detects hydrogen vibration  metal nanoparticles.
metal nanoparticles.
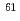 Ni synchrotron radiation-based M
Ni synchrotron radiation-based M ssbauer spectroscopy of nickel-based nanoparticles with hexagonal structure
ssbauer spectroscopy of nickel-based nanoparticles with hexagonal structureMasuda, Ryo*; Kobayashi, Yasuhiro*; Kitao, Shinji*; Kurokuzu, Masayuki*; Saito, Makina*; Yoda, Yoshitaka*; Mitsui, Takaya; Hosoi, Kohei*; Kobayashi, Hirokazu*; Kitagawa, Hiroshi*; et al.
Scientific Reports (Internet), 6, p.20861_1 - 20861_8, 2016/02
Times Cited Count:10 Percentile:40.59(Multidisciplinary Sciences)Ara, Kuniaki; Sugiyama, Kenichiro*; Kitagawa, Hiroshi*; Nagai, Masahiko*; Yoshioka, Naoki*
Journal of Nuclear Science and Technology, 47(12), p.1171 - 1181, 2010/12
Times Cited Count:10 Percentile:54.45(Nuclear Science & Technology)A study was conducted on the control of the chemical reactivity of sodium utilizing the atomic interaction between sodium and nanoparticles. The authors reported in a previous paper that the atomic interaction between sodium and nanoparticles increases and has the potential to suppress chemical reactivity. In this paper, the authors examined the released reaction heat and the reaction behavior. As a result, it was confirmed that the released reaction heat and the reaction rate decreased. From the results of experimental studies, it is clear that the suppressions of chemical reactivity are caused by a change in the sodium evaporation rate and fundamental physical properties such as surface tension which originate in the change in the atomic interaction between sodium and nanoparticle atoms. The suppression of chemical reactivity applying to FBR coolant was estimated for the case of sodium combustion and sodium-water reaction. It was confirmed that the concept of suspending nanoparticles into sodium has high potential for the suppression of chemical reactivity. Applicability as coolant to the FBR was investigated, including not only the chemical reaction properties but also the aspects of heat transfer and operation.
Ara, Kuniaki; Sugiyama, Kenichiro*; Kitagawa, Hiroshi*; Nagai, Masahiko*; Yoshioka, Naoki*
Journal of Nuclear Science and Technology, 47(12), p.1165 - 1170, 2010/12
Times Cited Count:11 Percentile:57.24(Nuclear Science & Technology)A study on the chemical reactivity control of sodium utilizing the atomic interaction of sodium with suspended nanoparticles was carried out. The atomic interaction between nanoparticles and sodium atoms were estimated by theoretical calculations and verified by fundamental physical properties measurements. Results showed the atomic bond of the sodium atom and the nanoparticle atom was significantly larger than that of the sodium atoms, when the transition metals that have the property of large electronegativity are applied as nanoparticles. From the theoretical calculation results, it was suggested that charge transfer occurs from the sodium atom to the nanoparticle atom. The fundamental physical properties of sodium with suspended nanoparticles were examined in comparison with that of sodium to verify the change of the atomic interaction. From the experimental results, it became clear that the surface tension becomes larger and the evaporation rate becomes smaller. These changes in fundamental physical properties were measured to verify the stability of the atomic interaction under the conditions of wide temperature range and the phase transformation from solid phase to liquid phase.
Saito, Junichi; Ara, Kuniaki; Sugiyama, Kenichiro*; Kitagawa, Hiroshi*; Nakano, Haruyuki*; Ogata, Kan*; Yoshioka, Naoki*
Proceedings of 16th International Conference on Nuclear Engineering (ICONE-16) (CD-ROM), 4 Pages, 2008/05
no abstracts in English
Saito, Junichi; Ara, Kuniaki; Sugiyama, Kenichiro*; Kitagawa, Hiroshi*; Oka, Nobuki*; Yoshioka, Naoki*
Proceedings of 15th International Conference on Nuclear Engineering (ICONE-15) (CD-ROM), 5 Pages, 2007/04
Liquid sodium is used as the coolant of the fast breeder reactor (FBR), because of its high thermal conductivity and wide temperature range of liquid. However the chemical reactivity with water and oxygen of sodium is very high. So an innovative technology to control the reactivity is desired. The purpose of this study is to reduce the chemical reactivity of liquid sodium by dispersing the nanometer-size metallic particles into liquid sodium. Sub-themes of this study are nanoparticles production, evaluation of reaction control of liquid sodium, and feasibility study to FBR. In this paper, we describe the research program of them.
Azuma, Shunichi*; Ishii, Hiroshi*; Asai, Yasuhiro*; Kitagawa, Yuichi*; Wakita, Hiroshi*; Yamauchi, Tsuneo*; Asamori, Koichi
Geodynamics of Atotsugawa Fault System, p.173 - 179, 2007/00
no abstracts in English
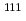 Cd knight shift in superconducting Cd
Cd knight shift in superconducting Cd Re
Re O
O ; Evidence for spin-singlet pairing
; Evidence for spin-singlet pairingSakai, Hironori; Tokunaga, Yo; Kambe, Shinsaku; Kitagawa, Kentaro*; Murakawa, Hiroshi*; Ishida, Kenji*; Ono, Hiroyuki*; Kato, Masaki*; Yoshimura, Kazuyoshi*; Walstedt, R. E.
Journal of the Physical Society of Japan, 73(11), p.2940 - 2943, 2004/11
Times Cited Count:8 Percentile:47.63(Physics, Multidisciplinary)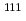 Cd NMR measurements have been performed in the superconducting (SC) state of the pyrochlore Cd
Cd NMR measurements have been performed in the superconducting (SC) state of the pyrochlore Cd Re
Re O
O at T
at T
 1 K, and in a field of less than 3 kOe below
1 K, and in a field of less than 3 kOe below 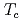 . The upper critical field at 0.1 K has been determined to be 4 kOe from in situ measurements of the ac susceptibility. A reduction of the Knight shift in the SC state is confirmed. The present results provide strong evidence that this compound has a singlet SC pairing symmetry.
. The upper critical field at 0.1 K has been determined to be 4 kOe from in situ measurements of the ac susceptibility. A reduction of the Knight shift in the SC state is confirmed. The present results provide strong evidence that this compound has a singlet SC pairing symmetry.
Saito, Junichi; Ara, Kuniaki; Sugiyama, Kenichiro*; Kitagawa, Hiroshi*; Nakano, Haruyuki*
no journal, ,
no abstracts in English
Saito, Junichi; Ara, Kuniaki; Ogata, Kan*; Fukunaga, Koichi*; Oka, Nobuki*; Kitagawa, Hiroshi*; Yamauchi, Miho*
no journal, ,
no abstracts in English
Saito, Junichi; Ara, Kuniaki; Fukunaga, Koichi*; Ogata, Kan*; Nagai, Masahiko*; Oka, Nobuki*; Kitagawa, Hiroshi*; Yamauchi, Miho*
no journal, ,
no abstracts in English
Saito, Junichi; Ara, Kuniaki; Nagai, Masahiko*; Fukunaga, Koichi*; Kitagawa, Hiroshi*; Yamauchi, Miho*; Oka, Nobuki*
no journal, ,
no abstracts in English
Kofu, Maiko; Hashimoto, Naoki*; Akiba, Hiroshi*; Kobayashi, Hirokazu*; Kitagawa, Hiroshi*; Tyagi, M.*; Faraone, A.*; Copley, J.*; Lohstorh, W.*; Iida, Kazuki*; et al.
no journal, ,
no abstracts in English
Saito, Junichi; Ara, Kuniaki; Sugiyama, Kenichiro*; Kitagawa, Hiroshi*; Yamauchi, Miho*; Yamashita, Akihiro*; Oka, Nobuki*; Yoshioka, Naoki*
no journal, ,
no abstracts in English
Ara, Kuniaki; Saito, Junichi; Sugiyama, Kenichiro*; Kitagawa, Hiroshi*; Ogata, Hiroshi*; Toda, Mikio*; Yoshioka, Naoki*
no journal, ,
no abstracts in English
Saito, Junichi; Ara, Kuniaki; Nagai, Keiichi; Nishimura, Masahiro; Onojima, Takamitsu; Sugiyama, Kenichiro*; Zhang, Z.*; Kitagawa, Hiroshi*; Nakano, Haruyuki*; Oka, Nobuki*; et al.
no journal, ,
no abstracts in English
Saito, Junichi; Ara, Kuniaki; Kitagawa, Hiroshi*; Nakano, Haruyuki*
no journal, ,
no abstracts in English